EXPOSING DEVELOPERS WHO FAIL TO APPRECIATE AND RESPECT COMMUNITY, ENVIRONMENT AND SPIRIT OF THE LAND
Port of Melbourne Authority Bay Dredging
Port Phillip Bay (Nerm) and Yarra River (Birrarung)
May08: The Yarra River above major sewer trunkline gets dredged - highly toxic sludge then dumped in 'spoils grounds' in the Bay. Bay dredging backers in background.
Toxic Nightmare!
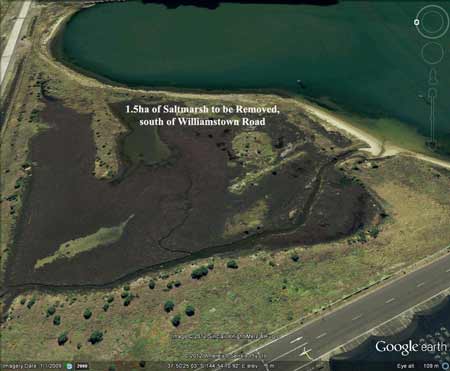
Ecology Out of Whack: Native Sea Urchins Threaten Kelp Beds South of Williamstown
September 6 2014: 70 Metre Seawall to be Built at Portsea
"Wave motion which occurs during high tides & north westerly winds in winter (4-5 weeks of the year) appears to be causing the major erosion problems at Portsea"
July 30 2014: Labor Floats Plan for Giant Pier out into Port Phillip Bay in its Bay West Port Plan
Labor is considering building a giant, hook-shaped pier stretching about eight kilometres into the sea as a way to make good on its proposal to build a new container port in the west of Port Phillip Bay near Geelong. The pier would extend at least three kilometres out to sea into deeper waters, which would reduce the huge amount of dredging needed to build the port, then run parallel to shore for about five kilometres. Large ships carrying up to 10,000 containers would dock at the offshore terminal to unload their cargo, which would be moved to land by rail.
July 29 2014: Sand Fence Slows Peninsula Erosion at Portarlington
July 7 2014: Bathing Boxes in Mt Martha Take A Battering As Coastline Continues to Erode After Wild Weather
June 24 2014: New Study into Portsea Waves
May 29 2014: Proposed Privatisation of Port of Melbourne Australia
April 10 2014: Dredging Blow to Port Plan
Port Phillip Bay faces massive dredging on a scale never before attempted in Australia, at a cost of billions, if Labor follows through on its policy to build a new container port between Avalon and Geelong, an investigation by the Transport Department has found. Building a port at Bay West would require dredging of between 66 million and 84 million cubic metres of material, including rock, from the sea floor - three to four times more than was dredged in the previous channel deepening project - the report found. This would be so ''technically difficult and prohibitively expensive'' it might ''prove not to be feasible''... Dredging would also be required at Port Phillip Heads and in the south channel, risking ''sea level rises in the bay (at least two centimetres); potential impacts on the marine national park at the heads; and increased navigational safety risks'', the briefing to Ports Minister David Hodgett says.
March 5 2014: Proceeds from port lease sale to fund transport projects
March 5 2014: Port Of Melbourne to be Sold regardless of who wins next State Election
Jan 2014:Erosion at Point Richards (East) Portarlington
Oct 7 2013: Investors keen for berth at Port
Sep 12 2013: Erosion Threatening Melbourne's Beaches
September 10 2013: Concern for Future of Peninsula Beaches
September 4 2013: Beach Boxes No Longer Suitable for Eroding Coastline in Mt Eliza
August 6 2013: Rock Wall to Protect Portsea Front Beach
July 14 2013: Portsea Retains Sandbag Wall Against Expert Advice
More than 10,000 cubic metres of sand was also transported from Gippsland, but immediately swept out to sea or dumped on neighbouring Shelley Beach to the north...The dredging of a rocky outcrop known as the Plateau, near the entrance of Port Phillip Bay, had increased tidal swells at the southern end of the peninsula by up to 70 milimetres, he said. ''The Plateau acted as a speed hump, but now storm surges are entering the bay relatively unimpeded and appear to have been reflected towards Portsea.''
July 7 2013: Beach Loss Linked To Dredging
May 27 2013: Bay of Plenty Looms As The Young Get Snapping
"The early catch in this year's research generated a sense of excitement, as well as relief. When this year's survey was completed, 322 baby snapper had been captured. The figures represented a reversal from the previous seven years when snapper spawning either failed or had only average success."
December 23 2012: Pier Review Finds A Nasty Threat To Our Shellfish Ways
December 9 2012: Bay Dredging in $1.2billion Dock Expansion
November 14 2012: Victorian Dredging Project Needs Review
(also see Radioactive Yarra?)
November 14 2012: Victorian Dredging Project Needs Review
Aug 8 2012: Portarlington Beach Renourishment Works Begin
May 28 2012: Fisheries Scientists Snapper Happy On The Job
Port Phillip Bay is ''the key spawning ground'' for the adult snapper population stretching from Wilsons Promontory to South Australia (known as the ''western stock''). ''The spawning goes on in the bay every spring. The bay is the nursery ground for the little juveniles. And as these juveniles grow up, they start to disperse and they move out along the coast and into Western Port. And they basically replenish the whole fishery,'' he says. ''The bay is the breeding ground that is most important for that whole fishery, so it's a crucial area and if it fell over we'd have major problems with the whole of that stock - which would affect a lot of anglers and fishermen along the coast.'' Snapper come into the bay to spawn in massive numbers every year. Dr Hamer says they start arriving about late September, but the biggest rush is probably through October and November.
January 30 2012: Bay Beach Problems Roll On With Algae Warning
December 16 2010: Continuing Anger Over Portsea Beach Erosion
December 15 2010: Will Portsea Beach Erosion Affect Tourism?
December 8 2010: Portsea Protection
November 27 2010: State Election. Voters Head to the Polls in Mornington and Nepean
"Lack of public transport, ambulance waiting times and the beach erosion at Portsea Front Beach – which the Liberals and the Greens accuse the Labor Government of having caused through dredging - will also be major issues for Nepean voters when they head to the polls".August 23 2010: Portsea Beach Battle
August 17 2010: Dredging Firm Fined For Oil Spill (The Age p5)
EPA Boskalis Enforceable Undertaking
The Dutch dredging firm that completed the $721 million deepening of Port Phillip Bay's shipping channels spilled 900 litres of hydraulic oil near Port Lonsdale in 2008. The spill was revealed yesterday in a settlement reached between the state government's Environment Protection Authority and Dutch dredger Boskalis. The spill occurred on August 30, 2008, near the entrance of the bay. Approximately 900 litres of hydraulic oil poured out of the vessel Queen of the Netherlands while it was dredging at Port Phillip Heads, near Port Lonsdale.
The EPA, in an "enforceable undertaking" agreement released yesterday, found that a "quantity of oil having toxic or otherwise dangerous characteristics" had come from the Quenn of the Netherlands. Boskalis has agreed to pay $75,000 to Swinburne University, as compensation. The money will be used to sponsor a PhD project at Swinburne, which will look at dredging and its environmental effects. Boskalis will also sponsor a training course at Swinburne on dredging. The oil spill and settlement costs will set Boskalis back $170,000.
June 17 2010: (Letter to Editor Age Newspaper)
I HAVE been a resident of Portsea for 13 years. I swim for five months of the year along Portsea's front beach. I use the beach daily. Somehow I feel more qualified to comment on the effects of storms and seasonal weather conditions than Environment Minister Gavin Jennings (''Renourishment plans for Port Phillip, Bellarine beaches'', The Age, 15/6). While the Brumby government is clearly unwilling to concede that channel deepening has affected beaches, it is laughable that the cause of beach degradation is blamed on environmental factors. Is Mr Jennings asking to be taken seriously when he asserts that ''we understand how Victorians love their beaches''. The evidence to the contrary is here for all to see as one of our great bay beaches is nourished with hessian sacks to hold back collapsing dune systems and protect properties. A repeat of Noosa Sound?June 7 2010: Shifting the problem (Letter to Editor Age Newspaper)
IT IS not only Portsea beach that is eroding suddenly and swiftly (''Life's not so swell on the beach at Portsea'', The Age, 5/6). Beaches towards the Heads (westwards of the pier) within Point Nepean have suffered dramatic erosion and exposure of middens also in the short time since dredging of the entrance. The Environmental Monitor claims that the beach "around the corner" (eastwards of the pier) is unchanged since channel deepening. However, that beach (Shelly Beach) is protected by Point Franklin, which effectively acts as a groyne. An unsightly rock groyne at Portsea might disperse the energy of the swell hitting the beach but will trigger reactions: sand trapped within the groyne will cause sand depletion elsewhere. Such effects can be seen at nearby Blairgowrie marina, where a huge spit of sand has been deposited within the marina over the 10 years since its construction, depleting nearby beaches; and at Queenscliff and Brighton harbour (to name three of many examples) where rock walls built to "solve" something are now having unwanted environmental impacts. The usual man-made interventions just keep shifting the problem. Jenny Warfe, Blue Wedges, DromanaErosion Portsea at access point to Portsea Front Beach - August 2010

Access to Portsea Front Beach July 2013

Portsea Front Beach July 2013 - Note size of sandbags.

July 2013: Access point to Portsea Front Beach
June 5 2010: Life's Not So Swell On The Beach At Portsea
Erosion Portsea Front Beach - August 2010

Erosion Control Portsea Front Beach - March 2012. Sea is rising 2mm per year around Australia.
May 5 2010: Portsea Beach Erosion Blamed On Bay Dredging
April 15 2010: Fake Beach To Cover 'Damage To Dredging'
December 13 2009: Quasi Terrorist Treatment for Tea and Biscuit Protesters
December 4 2009: Bay Dredging Winds Up
November 26 2009: More Dredge Work
November 26 2009: Labor Hails Dredging Success Says Bay Is Clean
November 25 2009: "The most recent report states that the abundance of sand flathead in the bay was significantly lower after 2007."
A statistical comparison between the mean sand flathead biomass in 2004 to 2007 and 2008 to 2009 indicates that the abundance is significantly lower in intermediate and deep water depths after 2007... there has been a 30% decrease in Port Phillip Bay as a whole, with a decrease in the Melbourne (46%) and Mornington (49%) regions. There was no decrease in the Geelong/Bellarine area...
November 21 - 27: Sea Level Rise Walk
September 25 2009: Port of Melbourne Authority & Decline in Sand Flathead in Port Phillip Bay
September 24 2009: After the Dredging
September 11 2009: Measuring The Fallout From The Port Phillip Bay Dredging Project
September 10 2009: Environment Groups Want Dredging Bond Spent
August 26 2006: Dredging Damage Mocks Premiers Praise
August 22 2009: Bay Project Dredges Up Unanswered Questions
August 21 2009: ABC Stateline Will the Dredging of Port Phillip Bay Live Up To Government Promises?
August 21 2009: Job Done Dredger Ready To Leave
August 6 2009: Erosion Causing Havoc On Bellarine Peninsula
June 21 2009: Controversy Settles in the Dredges Wake
May 26 2009: Plume of filthy dredge water at Webb Dock East, one hour after dredging by Cornelis Zanen. What impact will years of dredging plumes have on the health of the bay?

Lower Yarra getting a work over by Boskalis
April 29 2009: Winning awards and wreaking havoc (Melb Age Letters)
MY COLLEAGUES and I were shocked to hear that Ports Minister Tim Pallas has been awarded the 2009 environmental award from the Australian Marine Environment Protection Association. Perhaps, we mused, it's in the bungler category? But AUSMEPA's website says that Mr Pallas will receive the award "in recognition of the achievements of EPA Victoria and Melbourne Water in dramatically improving the water quality of Port Phillip and Victoria's coastal waters". Are these people fair dinkum? Others have commented that Mr Pallas should get an award for vandal of the year for his proposals as Roads Minister to build a freeway near Heide Museum and Park, and bulldoze his way through Banyule Wetlands, Warringal Parklands, and Frankston's Pines Flora and Fauna Reserve. As for the bay, we still don't know why anchovy have missed a breeding season, or why the fish study in the Yarra can't find any mullet to test. Of course, we now have a six-square-kilometre toxic dump in the bay. As Ports Minister, and channel deepening champion, Mr Pallas has a lot to answer for. As to why he has just received an environmental award, we remain mystified. Jenny Warfe, Blue Wedges, DromanaMay 13 2009: Tidal Havoc
February 7 2009: Project Drags on But End in Sight
January 18 2009: Businesses fear nosedive as dredging ramped up
January 9 2009: Light dredge still operating near gas pipeline just down from Westgate Bridge.
December 23 2008: Third Ship To Speed Up Bay Dredging
October 27 2008: Heavy Metals Contaminate Yarra Fish
Fears grow over 'poisoned' Yarra
I wouldn't eat any fish caught out of the lower Yarra, but thousands of people do ("Revealed: river's poisonous catch", October 27).
With a bag limit of 40 fish per day (if different species are caught) one person could theoretically catch nearly 300 fish per week out of the Yarra.
Are any of these fish ending up in Melbourne restaurants?
The health issue gets more serious if you consider bay dredging, where thousands of tonnes of contaminated sediment dredged out of the Yarra has been dumped in an uncapped bund in Port Phillip Bay.
How many fish currently sold at Footscray Market have been caught near the dumping grounds?
Species such as snapper, whiting, garfish, flathead, gummy shark, not to mention pilchards and anchovies, could all be tainted by ex-Yarra River toxic sediments such as mercury and arsenic.
Can John Brumby ease the concerns of fish eaters that all of these fish are safe to eat. Reader Herald Sun October 28 2008
October 10 2008: Killer Whales and Dolphins Move in as Bay Fish Dwindle
August 1 2008: Dredging Fails First Bay Test
July 21, 2008: Sandridge Beach Port Melbourne. Strong winds from the south west after rainfall of 15mm in Melbourne meant turbidity levels near the beach of of 100+ NTU's, most likely caused by dredging. Filthy water was the result.

July 29, 2008: 'Light Dredging' continuing near main sewer pipeline in Yarra River just downstream from Westgate Bridge.

ditto
July 19 2008: There's Something Rotten In Our Bay
July 16 2008: Blue Wedges May Pay Costs
| Days since start of dredging | 164 |
| Cubic metres dredged from: South Channel | 2.8 million |
| Port of Melbourne Channel | 2.2 million |
| Bay Entrance | 333,000 |
| Uncontaminated clay from Yarra | 259,000 |
| Contaminated silts from Yarra | 225,000 |
| Total Dredged | 5.9 million |
| Dredge Target | 22.9 million |
July 14 2008: Bay Dredging Plume Has Spread Beyond The Limits ACF Report
July 5/6 2008: Fish Kills Reported on Lower Yarra River near Newport. People told not to eat fish. Blame for this incident later put on milky substance leaching from Newport Power Station but Yarra was filthy from dredging during this time)
July 16 2008: Boskalis Dredge Vessel Cornelis Zanen dredging under Westgate Bridge.

July 16 2008: Cornelius Zanen and Support vessel.

July 16: Suction pump? on Cornelis Zanen

July 18 2008: Another Boskalis barge vessel.
July 6 2008: Another load of toxic sediment makes its way to the Toxic waste dump in Port Phillip Bay. Round trip from Yarra to dumping ground probably takes 3-4 hours, meaning 5-6 spoil trips a day if dredging occurs on 24 hour basis.
June 6 2008: Royal Boskalis 'Dummy Spit'
May08: The Yarra River above major sewer trunkline gets dredged. Bay dredging backers in background.

May 30: Another load of toxic sediment makes its way to the toxic dumping ground in the middle of the bay.

May 08: Note colour of water pouring from 'grab bucket'. Imagine what will happen when the major dredging takes place, several metres deeper than what is occurring over main sewer pipe at the moment. Likely to stink to high heaven as was reported on Seven News May 18.

Dredging commences in the Yarra. Image courtesy of Blue Wedges & P.Crotty

May 08: The start of dredging near the main sewer trunkline

Dredging Up Industrial Disease March 17 2008
April 21 2008: Yarra River Toxic Dredging Starts
2.1 million cubic metres of toxic sludge from the Yarra River mouth to be dumped in the Bay!
March 23 2008. Note sediment plume drifting south from near Hobsons Bay Channel during a high pressure 'long spell'. Images courtesy of bluewedges
March 17 2008 Note sediment plume associated with toxic waste bund construction near Hobsons Bay
Send Garrett an email
May 18 08: Closer Monitoring Plan for Dredging
April 5 08: Dredging Starts at the Heads
Mar 3 08: Bulky Queen Leaves Bayside Seriously Browned Off
Dutch 'Death' Ship - Queen of the Netherlands arrives 29/1/08.

Rye Beach after trial dredging in August 2005. Note black crap! Imagine what will be washed onto beaches when full-scale dredging occurs!
More Trial Dredging Beach images Rye Beach August 2005 here
For a more thorough analysis of the Bay Dredging Issue please go to the Bluewedges website here
The Company employed to do the Port of Melbourne Corporations' "Dirty Work". Who are Royal Boskalis Westminster nv?
Who are the masterminds behind this "insane" plan? (PoMC)
also here Australian Council for Infrastructure Development & here
Toxic Nightmare
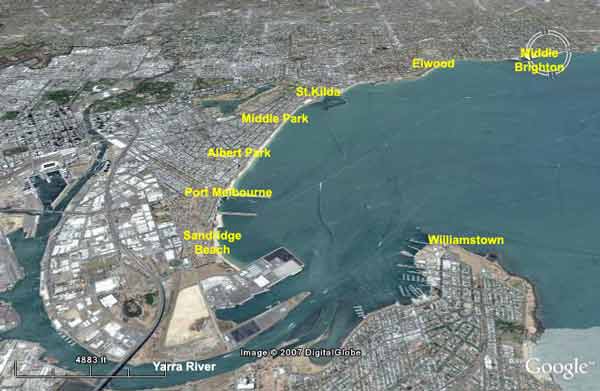
Beaches in Inner Melbourne likely to be most impacted by toxic plume from Yarra River Dredging (above). Sandridge Beach and Port Melbourne (Beacon Cove)likely to suffer the brunt of the toxic soup.


Yarra River downstream of Bolte Bridge. All of this area will be dredged.
2.1 million cubic metres of toxic sludge from the Yarra River mouth to be dumped in the Bay!
At Most Risk Beaches in 'Inner Melbourne'
Yarra River Concerns
Other threatened beaches here

Photo: The Age Craig Abraham (Sep 2005)
Bay Dredging Likey to Affect Beachgoers ( The Age 4/9/07)

graphic showing where the bay will be dredged
Tentative Timeline for Dredging
1. Hobsons Bay/Northern Shipping Channel (Feb-Oct 2008 & May-August 2008)
2. Dredging the Yarra (Jun - Aug 2008 (Toxic Spoil in Yarra) & Jan - May 2009 ("non-toxic" spoil in Yarra)
3. Dromana to the Heads (April 2009 - Jan 2010)
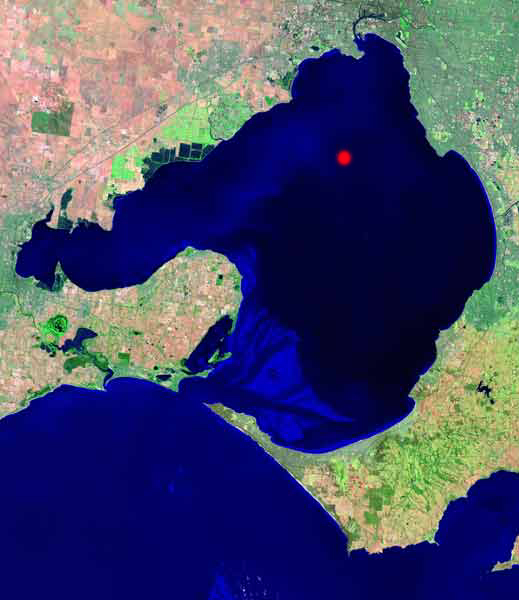
1996 CSIRO image of Yarra Plume Image (below)

"This image, taken from the cover of a 1996 CSIRO Report, shows a turbid flood plume from the Yarra. It can be seen that the plume extends from Williamstown to beyond Ricketts Point. If the plume contained toxic sediments from dredging, the bay and beaches from Williamstown to beyond Ricketts Point could be affected. Fishing and swimming may have to be banned. Currents in the bay are driven by wind, so that depending on wind direction, plumes from the Yarra can go down the east side of the bay, or across to the west past Altona."
Estimated contaminated spoil plume Yarra River
ANZECC Fresh Water (Yarra) Trigger levels here
ANZECC Marine Trigger levels here
EPA map of Yarra Estuary Here (90% ANZECC Trigger Level) here
Toxicants included in sediment plume from Yarra River dredging most likely to include;

(Please note: Radionuclides were not looked for by PoMC. In fact PoMC used an old study which indicated very low radionuclide levels, but the sampling sites were nowhere near the ship channels! Cobalt 60 and Caesium 137 could be expected to be found in the river channel, and there could be hotspots elsewhere, the legacy of visits from nuclear-powered ships. Dioxins were not looked for either, though bound to be there).
Inorganics: Antimony, Arsenic, Barium, Beryllium, Cadmium, Chromium, Cobalt, Copper, Lead, Nickel, Selenium, Silver, Tin, Zinc, Mercury, Total Cyanide, Ammonia as N, Nitrite + Nitrate as N (Sol.), Total Kjeldahl Nitrogen as N, Sulphate as SO4 2-, Sulphur - Total as S (LECO), Fluoride, Sulphide as S.
Organics: Total Organic Carbon, Tributylin, TPH C6 - C9 Fraction, TPH C10 - C14 Fraction, TPH C15 - C28 Fraction, TPH C29 - C36 Fraction, TPH C10-C36 Fraction, Benzene, Toluene, Ethylbenzene, meta-¶-Xylene, ortho-Xylene.
OCPs (Organochlorine Pesticides): Aldrin, alpha-BHC, beta-BHC, delta-BHC, 4.4'-DDD, 4.4'-DDE, 4.4'-DDT, DDT(Total), Dieldrin, alpa-Endosulfan, beta-Endosulfan, Endosulfan sulfate, Endosulfan, Endrin, Endrin aldehyde, Endrin ketone, Heptachlor, Heptachlor Epoxide, Hexachlorobenzene (HCB), gamma-BHC, Methoxychlor, cis-Chlordane, trans-Chlordane, Total Chlordane.
PCBs: Total PCBs, Aroclor 1016, Aroclor 1221, Aroclor 1232, Aroclor 1242, Aroclor 1248, Aroclor 1254, Aroclor 1260.
PAHs: 3-Methylcholanthrene, 2-Methylnaphthalene, 7.12-Dimethylbenz(a)anthracene, Acenaphthene, Acenaphthylene, Anthracene, Benz(a)anthracene, Benzo(a)pyrene, Benzo(b)fluoranthene, Benzo(e)pyrene, Benzo(g.h.i.)perylene, Benzo(k)fluoranthene, Chrysene, Coronene, Dibenz(a.h)anthracene, Fluoranthene, Fluorene, Indeno(1.2.3.cd)pyrene, N-2-Fluorenyl Acetamide, Naphthalene, Perylene, Phenanthrene, Pyrene, Total PAH.
Phenols: 2-Chlorophenol, 4-Chloro-3-Methylphenol, m-Cresol, o-Cresol, p-Cresol, 2.4-Dichlorophenol, 2.6-Dichlorophenol, 2.4-Dimethylphenol, Hexachlorophene, 2-Nitrophenol, 4-Nitrophenol, Pentachlorophenol, Phenol, Tetrachlorophenol, 2.4.5-Trichlorophenol, 2.4.6-Trichlorophenol.
OPPs (Organophosphorus Pesticides): Bromphos-ethyl, Carbophenothion, Chlorfenvinphos (E), Chlorfenvinphos (Z), Chlorpyrifos, Chlorpyrifos-methyl, Demeton-S-methyl, Diazinon, Dichlorvos, Dimethoate, Ethion, Fenamiphos, Fenthion, Malathion, Azinphos Methyl, Monocrotophos, Parathion, Parathion-methyl, Pirimphos-ethyl, Prothiofos
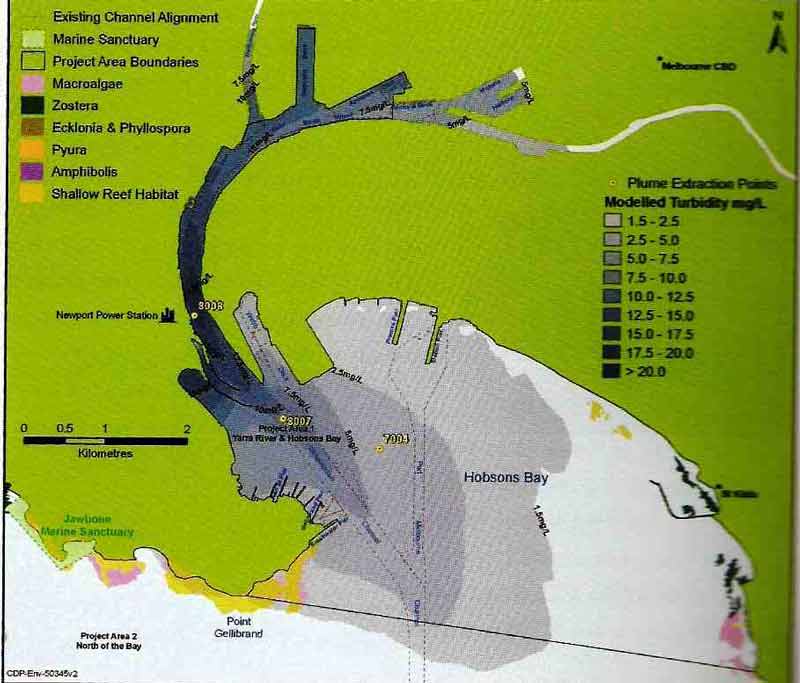
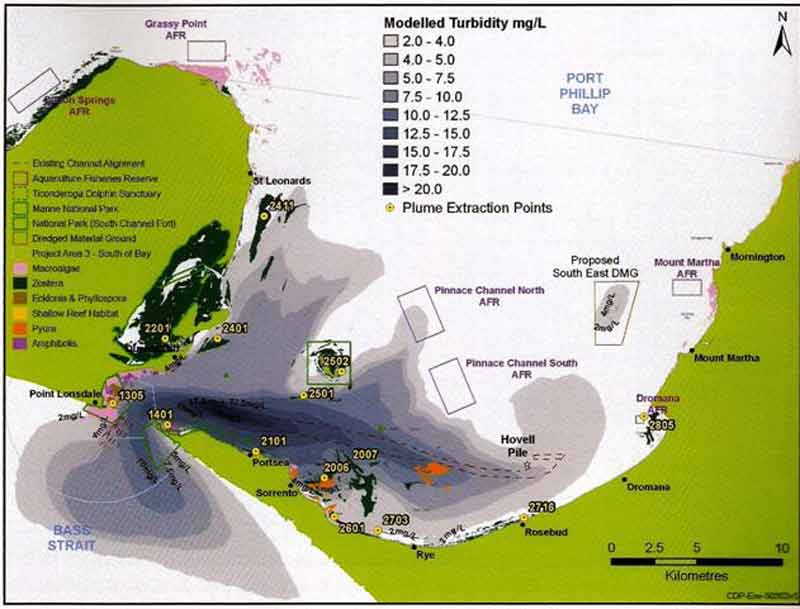
Estimated spoil plume at Port Phillip Bay heads
Summary of Predicted Fauna data
1. = Yarra B, 2. = Altona B, 3. = St.Kilda, 4. = Hobsons Bay, C 5. = North Melbourne Channel
| USEPA | MRL | Mussel | Mullet | Snapper | Flathead | |
| Organics ug/kg | ||||||
| Dioxins & dioxin-like PCBs | 2.56-04 | - | - | - | - | |
| Total PCB | 20 | 500 | 18.2 | 55.7 | 61.4 | 128 |
| Total PAH | 5.5 | - | 2855 | 64.6 | 45.02 | 49.79 |
| Total DDT | 117 | 1000 | 60.3 | 209 | 227 | 483 |
| Endosulfans | 24000 | - | 0.11 | 0.04 | 0.04 | 0.02 |
| Dieldrin | 2.5 | 100 | 3.38 | 4.5 | 5.93 | 3.64 |
| Total Chlordane | 114 | 50 | 1.47 | 4.15 | 5.13 | 8.18 |
| Total Dichlorphenol | 4.69 | 0.28 | 0.28 | 0.16 | ||
| Total Nitrophenol | 3.69 | 0.04 | 0.03 | 0 | ||
| Total Cresols | 8.25 | 0.08 | 0.06 | 0.02 | ||
| Phenol | 6.76 | 0.03 | 0.03 | 0.01 | ||
| Metals mg/kg | ||||||
| Tributyl Tin as Sn | 1.2 | |||||
| Arsenic Inorganic | 0.026 | 2 | ||||
| Cadmium | 4 | 2 | 0.95 | 0.16 | 0.24 | 0.07 |
| Lead | 0.5 | |||||
| Mercury | 0.4 | 0.5 |
| USEPA | MRL | Mussel | Mullet | Snapper | Flathead | |
| Organics ug/kg | ||||||
| Dioxins & dioxin-like PCBs | 2.56-04 | - | - | - | - | |
| Total PCB | 20 | 500 | 14.6 | 48.4 | 55.4 | 114 |
| Total PAH | 5.5 | - | 2237 | 44.8 | 33.45 | 34.59 |
| Total DDT | 117 | 1000 | 53.6 | 179 | 202 | 422 |
| Endosulfans | 24000 | - | 0.06 | 0.02 | 0.02 | 0.01 |
| Dieldrin | 2.5 | 100 | 2.13 | 2.81 | 3.75 | 2.31 |
| Total Chlordane | 114 | 50 | 1.34 | 3.67 | 4.69 | 7.38 |
| Total Dichlorphenol | 2.5 | 0.16 | 0.15 | 0.09 | ||
| Total Nitrophenol | 2 | 0 | 0.02 | 0.01 | ||
| Total Cresols | 4.29 | 0.03 | 0.03 | 0.02 | ||
| Phenol | 3.5 | 0.02 | 0.02 | 0.01 | ||
| Metals mg/kg | ||||||
| Tributyl Tin as Sn | 1.2 | |||||
| Arsenic Inorganic | 0.026 | 2 | ||||
| Cadmium | 4 | 2 | 0.54 | 0.1 | 0.13 | 0.04 |
| Lead | 0.5 | |||||
| Mercury | 0.4 | 0.5 |
| USEPA | MRL | Mussel | Mullet | Snapper | Flathead | |
| Organics ug/kg | ||||||
| Dioxins & dioxin-like PCBs | 2.56-04 | - | - | - | - | |
| Total PCB | 20 | 500 | 14.3 | 46.7 | 54 | 110 |
| Total PAH | 5.5 | - | 2002 | 38.26 | 29.13 | 29 |
| Total DDT | 117 | 1000 | 52.1 | 172 | 196 | 408 |
| Endosulfans | 24000 | - | 0.05 | 0.02 | 0.02 | 0.01 |
| Dieldrin | 2.5 | 100 | 1.7 | 2.25 | 2.98 | 1.84 |
| Total Chlordane | 114 | 50 | 1.29 | 3.51 | 4.52 | 7.1 |
| Total Dichlorphenol | 2.1 | 1.3 | 0.11 | 0.06 | ||
| Total Nitrophenol | 1.61 | 0.01 | 0.01 | 0 | ||
| Total Cresols | 2.77 | 0.03 | 0.02 | 0.01 | ||
| Phenol | 2.95 | 0.01 | 0.01 | 0 | ||
| Metals mg/kg | ||||||
| Tributyl Tin as Sn | 1.2 | |||||
| Arsenic Inorganic | 0.026 | 2 | ||||
| Cadmium | 4 | 2 | 0.45 | 0.08 | 0.11 | 0.03 |
| Lead | 0.5 | |||||
| Mercury | 0.4 | 0.5 |
| USEPA | MRL | Mussel | Mullet | Snapper | Flathead | |
| Organics ug/kg | ||||||
| Dioxins & dioxin-like PCBs | 2.56-04 | - | - | - | - | |
| Total PCB | 20 | 500 | 12 | 37.9 | 45.7 | 91.6 |
| Total PAH | 5.5 | - | 71.21 | 13.93 | 11.33 | 10.02 |
| Total DDT | 117 | 1000 | 44.5 | 141 | 168 | 342 |
| Endosulfans | 24000 | - | 0 | 0 | 0 | 0 |
| Dieldrin | 2.5 | 100 | 0.46 | 0.61 | 0.8 | 0.49 |
| Total Chlordane | 114 | 50 | 0.74 | 1.96 | 2.59 | 4.03 |
| Total Dichlorphenol | 0.64 | 0.03 | 0.03 | 0.01 | ||
| Total Nitrophenol | 0.52 | 0 | 0 | 0 | ||
| Total Cresols | 1.16 | 0.01 | 0.01 | 0 | ||
| Phenol | 0.95 | 0 | 0 | 0 | ||
| Metals mg/kg | ||||||
| Tributyl Tin as Sn | 1.2 | |||||
| Arsenic Inorganic | 0.026 | 2 | ||||
| Cadmium | 4 | 2 | 0.15 | 0.02 | 0.04 | 0.01 |
| Lead | 0.5 | |||||
| Mercury | 0.4 | 0.5 |
| USEPA | MRL | Mussel | Mullet | Snapper | Flathead | |
| Organics ug/kg | ||||||
| Dioxins & dioxin-like PCBs | 2.56-04 | - | - | - | - | |
| Total PCB | 20 | 500 | 2.08 | 6.4 | 7.88 | 15.7 |
| Total PAH | 5.5 | - | 71.21 | 1.02 | 0.83 | 0.68 |
| Total DDT | 117 | 1000 | 9.37 | 28.9 | 35.4 | 71.4 |
| Endosulfans | 24000 | - | 0 | 0 | 0 | 0 |
| Dieldrin | 2.5 | 100 | 0.03 | 0.04 | 0.05 | 0.03 |
| Total Chlordane | 114 | 50 | 0.06 | 0.17 | 0.22 | 0.34 |
| Total Dichlorphenol | 0.04 | 0 | 0 | 0 | ||
| Total Nitrophenol | 0.03 | 0 | 0 | 0 | ||
| Total Cresols | 0.07 | 0.01 | 0.01 | 0 | ||
| Phenol | 0.06 | 0 | 0 | 0 | ||
| Metals mg/kg | ||||||
| Tributyl Tin as Sn | 1.2 | |||||
| Arsenic Inorganic | 0.026 | 2 | ||||
| Cadmium | 4 | 2 | 0.01 | 0.02 | 0.02 | 0 |
| Lead | 0.5 | |||||
| Mercury | 0.4 | 0.5 |

The mouth of the Yarra River. Toxic Hotspot.
Yarra River Concerns
(Printed in Melbourne Age 3/11/07 written by Friends of the Earth Melbourne)
Ah, but the smell
I HOPE that all business proprietors and residents who live and work in close vicinity to the lower Yarra River are having a serious look at the dredging of the bay and the Yarra. The dredging plan will also see several kilometres of the river dredged up to Bolte Bridge. This dredging will release some of the most toxic substances known anywhere. It will most likely also release highly offensive smells.
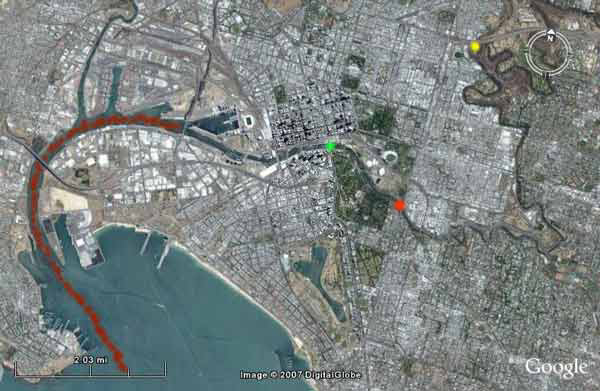
The Yarra is an estuary from Hobsons Bay to Dights Falls in Collingwood. Tidal currents could facilitate the movement of highly toxic and strong-smelling sediments upstream from Bolte Bridge, past the CBD for several kilometres. Friends of the Earth,
Aerial view of Yarra River. Yellow dot marks Dights Falls, which is where salt water and fresh water meet on the Yarra River. The green dot marks the Princes Bridge where a waterfall (until 1880) once marked the limit of salt water 'intrusion' into the Yarra River. The scarlet line indicates a rough line where the Yarra dredging will occur, ending at Bolte Bridge. The Yarra estuary extends for 22km from Hobsons Bay to Dights Falls. All of the estuary could be impacted. The red dot indicates the Punt Road Bridge.
Under certain conditions it is not inconceivable that sediment tainted water could flow upstream, carried by tidal currents and winds, past the CBD of Melbourne, possibly through to Dights Falls. What will be the odour caused by digging up toxic sediment that has laid at the bottom of theYarra River for up to 160 years? This scenario could well play out particularly in times of low flow on the Yarra - ie times of low rainfall (summer-autumn).The stench could be overwhelming for businesses and residents along the Yarra.
Melbourne Times November 7, 2007 p7
"Expert warns: bay dredging could cause stench by Bianca Hall
Dredging the Yarra and channels in Port Phillip Bay will stir up more than 160 years worth of industrial waste and create foul odours that will waft through the city, Richmond, Abbotsford and Hawthorn, according to Friends of the Earth.
Environmental researcher Anthony Amis has been examining the health of Melbourne's waterways for Friends of the Earth and says the impacts of dredging will extend far beyond the port areas.
Under a plan by the Port of Melbourne Corporation, given conditional approval by the Government last week, bay shipping channels and Hobsons Bay would be deepened to accomodate larger container ships.
But Mr Amis said while there had been a lot of debate about the effects of dredging on the bay, little attention had been paid to the health of the Yarra and its tributaries.
"Imagine the stench created by stirring up combined effluent of 160 years of industrialisation," Mr Amis said. "These sediments are contaminated with almost every toxic substance known to humanity."
The Yarra river up to Dights Falls is a 22km estuary, with a combination of salt and fresh water.
Mr Amis said water flowed both downstream and upstream, and salt water was confined by the falls at Abbotsford.
He said Yarra flows heading upstream, which were mainly affected by tides in the bay, increased during storms or sea rises at Williamstown. During these times, downstream flows in the Yarra heading towards the bay slowed down.
"It is not inconceivable that in times of low Yarra flows, particularly in autumn and summer, great amounts of recently dredged , foul-smelling toxic sediment could actually washe back up the Yarra River, past Crown Casino, past Southbank, past the Tennis Centre and ultimately all the way past South Yarra, Richmond and Hawthorn," Mr Amis said.

Yarra River near The Warmies north of Williamstown. At risk of toxic soup at 15-17.5mg/L at this location. The Yarra River estuary is also impacted by the water fluctuation of Port Phillip Bay. Sea level fluctuations can be as large as 0.8m at Williamstown over 3 days. Storms can also cause sea level fluctuations.
Dredging after-effects: rivers run through it
Stephen Cauchi Sunday Age November 18, 2007
PROPOSED dredging in Port Phillip Bay and in the Port of Melbourne could also create a stink in the Yarra and Maribyrnong rivers, with tidal surges forcing smelly sludge upstream as far as Abbotsford, say anti-dredging groups.
The Port of Melbourne said that its extensive modelling showed the problem would not eventuate, but Monash University says it might.
The Government has already admitted the $770 million dredging project, which is expected to start in January, could affect beaches all along the bay, including Port Melbourne and Williamstown.
However, the environmental group Friends of the Earth, which opposes channel deepening, said the toxic sludge unearthed in the dredging could wash upstream as far as Dights Falls, Abbotsford, because the Yarra is a tidal river.
A spokesman for Friends of the Earth, Anthony Amis, said that industrial refuse such as heavy metals and other toxins would be dredged up in the channel-deepening process and could easily wash upstream.
"The Port of Melbourne Authority claim that the toxic plume will only spread as far as William Street," said Mr Amis. "In times of low flow, sea water can go as far upstream as Dights Falls, (as) the Yarra estuary is actually 22 kilometres long.
How on earth will the sediment stop at William Street if it's flowing upstream on a strong tidal current?"
Mr Amis said that 3 million cubic metres of sediment would be dredged from the Yarra up to Bolte Bridge. "The sediment contains many heavy metals and other toxins (and) the smell of the exposed sediment could well be very nasty. I fear that these toxins may wash up back the river past the CBD into the Yarra near Hawthorn and Richmond."
Mr Amis said a 1982 paper published in the Australian Journal of Freshwater Research showed how far salt water from Port Phillip Bay could wash upstream, with salinity detected "beyond Bridge Road".
The paper, Water Movement and Salinity in the Yarra, stated that "the tidal influence in the Yarra and Maribyrnong estuaries is very dependent upon weather conditions … when a sudden increase in water level occurs at Williamstown as a result of storms in Port Phillip Bay, water is forced up both estuaries increasing water level in the estuaries".
A Port of Melbourne spokesman said the Yarra was "tidal, like any river" but "our modelling would not suggest that (a tidal plume)".

Yarra River near the Warmies. The flow pattern of bottom water can be quite different to surface water. Writing in 'Water Movement & Salinity in the Yarra and Maribyrnong Estuaries' (Australian Journal of Marine and Freshwater Ecology, 1982 33, 401-15)'; "For part of the time the bottom water moved upstream with a velocity as high as 24cm s-1, and at other times moved downstream with maximum velocities reaching 10cm s-1...During the survey there was a net movement of saline water upstream past Punt Road, corresponding to the general increase in water level... The tidal influence in the Yarra and Maribyrnong estuaries is very dependent upon weather conditions... It seems that when a sudden increase in water level occurs at Williamstown as a result of storms in Port Phillip Bay, water is forced up both estuaries increasing water level in the estuaries. Subsequently, as the level at Williamstown drops the water banked up in the estuaries is able to flow with high velocities out towards the bay...With medium river flows such as occurred on 12 July, the tip of the salt wedge penetrated to somewhere between Punt Road and Bridge Road - approximately 15km upstream from Hobsons Bay..."

October 7 2007: Blue Wedge rally under the West Gate Bridge near the Yarra River.

This portion of the Yarra River also to be dredged.

Yarra River Estuary at Bridge Road Hawthorn/Richmond under threat from dredging plumes carried by tides?

Prime fishing habitat near Williamstown (Point Gellibrand).

Rye Beach after trial dredging in August 2005

Rye Beach after trial dredging in August 2005
also, Middle Park Beach, Port Melbourne, Sandridge Beach, Williamstown Beach, Altona Beach
Send Garrett an Email
"Your Dreamworld is Just About to End"
Mar 28: Melbourne DredgingOpponents Lose Case
Feb 21: Dredge Protest Group Deal Bitter Blow
Feb 7: Garrett signs off on Environmental Management Plan
Jan 23: Garrett to Face Fresh Dredge Case 23/1/08
Send an email to Federal Environment Minister and ex lead singer of Midnight Oil Peter Garrett
"Slightly less virulent but just as tireless and more influential in the pop music culture are those who wield their "compassion" as a weopon against the middle class and its perceived politik. This mask of compassion makes the pathology sellable to that very middle class. Springsteen, U2, Midnight Oil, Sinead O'Connor, and Sting currently exploit this angle most effectively" p72 Rock and the Pop Narcotic Joe Carducci 1990

The Maireener Shell, Phasianotrochus Irisodontes, found only in the Southern seas of Australia and used by Aboriginal women for thousands of years, it is great cultural significance. Traditionally the shell was used as ornament, with strings broken apart in mourning. They were also used in trade for flint and ochres. The tradition of stringing shells continues in Tasmania today, with necklaces represented in most major museums and public galleries. They are valuable heritage items. See Lola Greenos work at Ian Potter Museum, Museum of Tasmania and the Powerhouse Sydney.
The Maireener shell is only found in limited locations, of the twelve recorded locations in Victoria three are to be found in the proposed dredge areas.
Phasianotrochus is a herbivore and grazer. They feed and live on the seagrasses Amphibolus and Posidonia. They are found at the low tide mark to a depth of five metres. At breeding time in summer they cover themselves in soft corals and migrate to deeper water to a depth of ten metres where they lay their eggs, which float for one or two days before anchoring themselves. They then migrate back in April. When they are found, they are found in abundance. They are an important source of food for Parrot fish and they live for one year.
The SEES report totally ignores the whole subclass Prosbranchia (Top shells) of which the Maireener is one. The loss of seagrass as habitat, food source and breeding ground as well as the effects of sediments and turbidity on the respiratory system and larvae stages of Phasianotrochus would be fatal.
To date the story of the Maireener on the land of the Boonerwrung and Wathaurung people is largely unknown, the deaths, disease and displacement that accompanied colonisation has been profound. Today cultural exchange workshops between Tasmanian and Victorian women are proposed. Research into necklaces of unknown origin is taking place.
THESE SHELLS SHOULD BE REGARDED AS NATIONAL TREASURES AND PROTECTED AS SUCH.
For more information contact Lindy at patchadwick@bigpond.com
Doctors Concerned About Yarra Dredging 11/3/08
New Review Planned for Dredging 27/2/08
National Blockade Hits Peakhour Traffic 19/2/08
Catastrophe Warning Over Channel Works 18/2/08
1000+ rally at Rosebud 18/2/08
No Compo From Dredging 12/2/08
Dredging Destroying Scallops 10/2/08
Protesters fined as dredging begins 8/2/08
State Bullied Over Port 2/2/08
Contract a Secret for Three Years 1/2/08
Don't Dredge the Yarra 30/1/08
$500 million bill for dredging 29/1/08
Blue Wedges on US watch list 27/1/08
It's Time to Dredge Up a Few Secrets 26/1/08
Toxic Shocker 24/1/08
Scientists Question Yarra Toxic Sediment 21/1/08
Bay Hit By Fish Health Scare 18/1/08
7.30 Report 8/1/08
Counter terrorism-police-seek-meeting-with-bay activists 16/1/08
D-Day for Bay as Last Legal Hurdle Falls 15/1/08
Call for Garrett to Halt Toxic Dumping 10/1/08
Channel Deepening Delay to Affect Bay Beaches 9/1/08
Baywatch Reveals Swarms of Unknown Life 9/1/08
Vigil as Port Phillip Bay Dredging Case Nears 9/1/08
Last Minute Challenge to Bay Dredging 6/1/08
Fruit of the Plume Leaves Portsea Paradise Lovers Fretting 6/1/08
In Depth Battle For The Bay 6/1/08
Bay dredge court fight to go ahead (age) 6/12/07
Dredging After-effects: rivers run through it (sunday age) 18/11/07
Protest hopes to give dredging big wedgie (age) 17/11/07
Dredge Threat to Beaches (sunday age) 11/11/07
Dredging impact could last 30 years (July31, 2007)
Toxic Plume May Reach Docklands
http://www.dolphinsofportphillip.com/
(www.portofmelbourne.com/portdev/channeldeep/ees.asp)
Dredging raises power fears by Jewel Topsfield - Sunday Age 14/11/04
Background on Dredging
The Port of Melbourne Corporation (PoMC) is asking Victorians to take a huge environmental and social gamble, merely so that a number of ships entering the Bay can load to full capacity.
The Southern waters of Australia, including Port Phillip Bay has the highest diversity of marine species anywhere in the world. 90% of species occur NOWHERE else on earth! Many of those species have not even been fully studied - and yet, before we understand our beautiful local underwater world, we are being asked to sanction its destruction - merely so that some larger ships can enter the Bay fully laden.
Scope of the Channel Deepening Proposal:
- .. 40 + million cubic metres of sand, silt, rock to be removed from the The Rip and sea bed (40,000,000 tonnes) and
- .. relocated to huge new spoil dumping grounds within the Bay
- .. thirty (30) times larger than any previous dredging project in the Bay
- .. At least 2 years duration - 24 hours a day, 7 days a week - noise, lights etc. a.. source of turbidity & sedimentation levels never before experienced in the Bay
- .. re-location of 5 essential services under Westgate Bridge ($60-80 Million taxpayer funded) so deeper draught ships can enter the Yarra River
- .. Re-location of Footscray Markets ($300 million - taxpayer funded), so Swanson Dock and storage facilities can be extended
What is at stake? The PoMC is sanguine about the 180+ threats in the proposed channel deepening - but their suite of consultants will have moved on well before the consequences hit!
129 "extreme" or "high" environmental threats are listed for our Bay - why should we be asked to consider ANY risks - let alone 129 serious and 54 moderate threats? Are we offered over 180 seriously or moderately good economic reasons to go ahead with the project? NO !
Here are some of the serious threats:
- .. Increased turbidity: fish, dolphins, seals, penguins, fish, corals etc. can't feed
- .. Loss of species we know very little about
- .. Re-suspension of nutrients, pollutants: dioxin, pesticides, insecticides, and other industrial contaminants, and heavy metals such as cadmium, mercury, and lead would re-enter the foodchain
- .. Reduced primary production from phyto- and zooplankton: less food source for higher order species, and less efficient Nitrogen cycling, leading to:
- .. Toxic algal blooms: Pea green water, stinky corners - $$$ to partially rectify - Dr. Graham Harris CSIRO Fellow advises that once the balance has been tipped no amount of rehabilitation will completely restore the Bay to its former condition...
- .. Drastically compromised water quality - $$$ to build new treatment plants Regulation?
The EES proposes Environmental Management via a complex "self-regulation" model - an alliance between the Proponent and the international, privately owned Dredging contractors. The EPA is relegated to an "advisory" role it seems. Has self-regulation been a great success for the environment in the past? This project is purely about moving boxes around the Nation.
- a.. It is a logistics puzzle, not complex science and
- b.. Melbourne Port will always be physically limited by Melbourne itself
- c.. Based on continued petty rivalry between states for the biggest container port!
- d.. Predicated on continued growth of trade, particularly imports- forecast that container trade through Victoria will quadruple by 2030 regardless of whether channel deepening proceeds (Pricewaterhouse Coopers 2003)
- e.. More container throughput has meant greater mechanisation and LOSS >of waterside jobs in other countries.
- f.. There is considerable community concern about increased beach erosion - particularly adjacent to dredged areas
Do we risk our Bay and beaches for a 19th Century parochial response to a 21st Century national issue?
So - What is the alternative?
- a.. Presently, only 30% of ships cannot fully load - but 70% can! Ships that can't could offload their excess at an existing NATURAL deepwater port -Brisbane, Sydney, Fremantle, Darwin - all connected to the National standard gauge rail
- b.. Funds for the channel deepening project could be allocated to a joint States project (a la Murray River) to further duplicate the national rail grid in a NATION building exercise - creating more sustainable jobs in regional areas as well
- c.. Melbourne could change its marketing focus to the preferred port for medium sized shipping to Australia
- d.. Supersized vessels cannot now use the Panama Canal - USA has responded by triplicating/quadrupling rail tracks across the Nation - and its working. If the Yanks can do it so can we!
Warren Ashdown
Executive Officer Association of Bayside Municipalities
Melbourne 12 August 2004
Dear Sir,
This letter conveys to you my final conclusions concerning the review of the final Port Philip Bay Environmental Effects Statement undertaken on your behalf.
General Impressions
I have now read most of the final EES documents for the proposed channel deepening of Port Philip Bay (PPB) and had a series of discussions with the project proponents and their consultants to clarify particular issues. My general impression is that these final documents are much improved from the preliminary documents released earlier in the year. Many of the points raised by my earlier criticisms have now been covered. Some of the underlying science is still incomplete but the state of the art in physical and ecological modelling, coupled with fundamental uncertainties in calibration and validation data ensure that it is simply not possible to eliminate all the potential uncertainties. The science required by this EES is at the forefront of environmental science - and while we can anticipate some of the potential risks, there will always be uncertainties. In particular it is not possible to predict all of the potential long term ecosystem impacts of the short term dredging program. The final EES is therefore set in a risk management framework - which is appropriate. Furthermore the EES acknowledges that not only are there risks in the carrying out of the project, but also that there are residual risks which cannot be mitigated by appropriate risk management in the context of an Environmental Management Plan (EMP). [It should be noted that the final EMP will not be agreed until after the Independent Panel has handed down its decision.] Society needs to have a debate about whether these residual risks are acceptable given the overall costs and benefits of the project.
Specific Comments
I do suugest that there could have been greater transparency in the documentation. It required detailed reading of about 30kgs of documents (equivalent on CD-ROM) to find some of the key statements. The summary document does not fully acknowledge the initial and residual risks - and like many other documents produced by the project proponents tends to put an optimistic gloss on the whole project. While the original key features summary reports and appendices were in many respects insufficient to determine if the necessary modelling and integration had been done, the new EES documentation is reasonably complete and does address many of the necessary issues. Most of the concerns raised in my previous report have been covered in the new documentation. Yes, there are still a few minor points that can be raised, but, overall, there is enough there now to be able to identify and assess most of the risks. The final EES does contain sufficient references to the scientific literature and is aware of the "state of the art" in various disciplines. The debate has moved a significant way forward since the initial documents. The EES does, I believe, put to rest any concerns about tides, changes in sea levels and wave height effects. I have never believed that these are likely to be major issues. Given the small effect overall on the geometry of the Heads (in terms of tidal exchange, a small deepening of an already modified shipping channel) then the changes to the physics of the Bay are not likely to have significant impact. The EES report uses the word "imperceptible" - I am not sure if that is the work I would have used but nevertheless, the effects are likely to be small. I am also of the opinion that the potential effects of oil spills, noise, lights at night, aesthetics etc. are also not major, and will be of short term impact. While there may well be significant short term effects (and a nuisance created), the EMP will be capable of managing these effects. Equally the EES discusses the potential risks of introducing more exotic species to the Bay and proposes suitable management actions. These are risks but they are, in my view, manageable.
Risks to the Bay as a whole
There is an important acknowledgement in the EES that there will be risks to the Bay as a whole (e.g. disturbance to the N cycle in the Bay as I predicted in my earlier paper) and that there will be risks to particular assets (e.g. National Parks at the Heads and Mud Island, diving, ecotourism) and species (e.g. sea grasses, penguins, dolphins, various species of fish etc.) There are, whether we like it or not, fundamental uncertainties around the long term implications and impacts of the short term insult to the Bay through dredging. Both the EES and the project proponents publicly acknowledge the uncertainties and risks and they are concerned about them. The final EES does now accept that there will be impacts on Bay-wide processes of Nitrogen cycling and denitrification and the figure arrived at (an effective increase in load of 250 tonnes of Nitrogen) is not significantly different from the estimates arrived at in my previous paper. There has been some debate about the possible impact of this change to the overall nitrogen cycle in the Bay. I still feel that this change is significant - at least during the period of dumping and for a while afterwards. There are risks here, especially if the dredging period happens to correspond to a period of higher than normal rainfall, and there remain residual issues about recovery times for benthic processes after they have been smothered by the dredge spoil.
The major impact of the Bay is going to be the effects on ecosystems, species and ecological processes arising from the suspended sediments stirred up by the dredging itself and disposal of the dredged material. In particular, there are long term unassessed risks to the National Park ecosystems at the Heads, to fish etc and to the sea grass beds in the Southern part of the Bay. These arise from smothering and light penetration reductions through plumes of sediments and the increased water turbidity. This is where there are acknowldged to be "extreme" or potentially "catastrophic" risks of long term damage to the Bay from the short term dredging activities. The possibility of hysteretic effects (points of no return) is real.
The EES speaks of regeneration and recovery times of up to 10 years - this may be optministic in the case of sea grasses in the Soutern part of the Bay. The final EES acknowledges that the base case modelling of the dredging indicated that risks to the ecosystems in the Southern part of the Bay were not acceptable. The reason for this is the fact that while tidal and wind driven currents are low in the Northern part of the Bay (and hence sediment plumes from dredging and dumping will not travel far), in the Southern part of the Bay, where tidal streams are strong, the sediment will be widely dispersed. The modelling to support the risk analysis in this area is not complete in the final EES but the proponents argue (and I accept their arguements) that the modelling is adeqaute to define some broad risk categories and guide the EMP. There always will be uncertainties in the generation of sediment plumes, their disposal and in the ecosystem impacts of the dredging activities. The problem we have is that it is not possible to collect data on the dredge (in terms of the generation and dispersal of the sediment plumes) until it is actually operating in the Bay. There are significant uncertainties surrounding the actual performance of the equipment in situ and this means that any predictive modelling will inevitably be fraught with uncertainty. Dredging the shipping channels in the Great Sands and through the Heads therefore poses significant risks to the ecosystems, seagrasses, biodiversity and fish (and the National Parks) around the Heads. I acknowledge that a risk management framework takes care of this - and relies as best we can on expert judgements (indeed the models are no more than codified expert judgements). I do also acknowledge that there are fundamental uncertainties in the science in this area so that even further improving the models would leave significant risks. Given this, the hydrodynamic and ecosystem modelling is adequate to the task (not perfect, but fit enough for purpose). So we probably have enough to go on with the reports we have. There are, no matter what, residual uncertainties in all this. Therefore, it is acceptable for the EES to categorize this as an area of significant risk both to the environment and to the project.
The EES asserts that the proposed Environmental Management Plan (EMP) and an adaptive management regime will suffice to reduce these to "moderate" or manageable proportions. The precise natures of the EMP and of the adaptive management plan are yet to be fully determined. As David Fox has pointed out, the risk assessment framework used, whilst adhering to Australian Standards 4360 methodology, does treat the potential risks one by one. The specialists employed were asked to rate the likelihood and the consequences of risk for each ecosystem or species - penguins, fish and seagrasses - seperately and did not necessarily use commensurate scales. Furthermore it seems that some of the extreme risks were rated as only "moderate" on the basis of being extremely unlikely. What increases the overall risk of ecosystem damage is lack of integration of associated risks - e.g. if we lose the seagrass beds then we will also lose the associated fish that require seagrasses for recruitment; and so on. This takes the discussion into the region of cumulative impacts - a difficult area for which there are methods.
Conclusions
The risk assessment and risk management framework used in the final EES accepts that there will be residual risks and identifies them. The greatest long term risks are to key ecological assets in the Southern part of the Bay. It is here that we have National Parks, high biodiversity, key species, high aesthetic values and eco-tourism activity.
In short the focus must now be on:
*Do the benefits of the proposed project exceed the acknowledged risks - in particular the "residual" risks and the uncertainties? The National Parks around the Heads and at Mud Island seem to be particularly at risk. Is this acceptable? Because there is no definitive scientific answer to this question, it is debate that society must have.
*Is the precise form of the proposed EMP sufficient to reduce the base case risks to acceptable levels? Will adaptive management of the dredging serve to mitigate the evident risks? In my view we need more transparency and debate on this issue. For now, we have to take a lot as read in this regard.
*If the project goes ahead, what kind of monitoring program (and technology) can provide the kind of information that will be required on a day to day basis to manage the dredging project from an environmental point of view? What form of public scrutiny, disclosure or transparent audit process can we then put in place to give comfort to those who doubt that the project can proceed without acceptable risk?
There is little more than science can do to resolve these issues other than to provide "decision support" to the community in the form of an ongoing monitoring and adaptive management framework should the project go ahead. The final EES recognizes that there are risks associated with the project - it is now a matter for the Melbourne community to decide if, on the balance of probabilities, the project is worthwhile.
Graham Harris CSIRO Fellow Hobart, Tasmania.
Dredging raises power fears by Jewel Topsfield - Sunday Age 14/11/04
The Newport power station, a major contributor to the national electricity grid, could be forced to shut down during the proposed $545 million Port Phillip Bay channel deepening project.
Operator Ecogen Energy says an environmental effects statement on the project does not identify the risks to the power station, which supplies electricity during peak demand periods such as heatwaves.
The 500-megawatt station on the west bank of the Yarra River pumps water from near the mouth of the river to cool its turbines, then returns warmed water to Hobsons Bay.
Ecogen Energy general manager John Edelsten said if the water contained contaminated sediment dredged up during the channel deepening, the Environment Protection Authority could revoke the power station's operating licence.
The EPA licence specifies limits for temperature, flow, acidity, iron, ammonia, chlorine, carbon, nitrate, turbidity and dissolved oxygen.
Mr Edelsten also said if the sediment contained high levels of sulphide it could corrode tubes in the power plant, forcing the station off line. "These are all unknowns. That is why we are so concerned about all of this and seek assurances that these things won't impact on us," he said.
If the station were shut down there would be power shortages in peak periods and that could lead to blackouts across the state, he said.
The $12 million environmental effects statement, which found that the dredging of Port Phillip Bay's channels would have no long term ill effects, is the biggest undertaken in Victoria.
But an Ecogen submission to a Government-appointed panel says the study does not cover the potential effects on the power station.
Power blackouts could have serious economic effects on the state, it says. "One only need consider the impacts of the Longford gas explosion."
The submission raises concerns that fish at one of Melbourne's most popular angling spots, known as "the Warmies", could become contaminated as a result of the dredging stirring up polluted sediment. The Warmies is the colloquial name for the power station's cooling water outlet, where warm current expelled from the plant attract shoals of tailor fish.
Ecogen's submission said the risk of fish contamination required further investigation. "In the event of any risk being posed to thrid parties, Ecogen would request indemnification from the state," the submission says. "We ask the panel to call upon the Government, via its Port of Melbourne Corporation, to take all necessary steps to ensure that none of the identified occurrences which would jeopardise the operations of Newport Power Station come to pass."
Mr Edleston said Ecogen was not opposed to the channel deepening provided there were proper controls.
Past reports
P110-112 The Politics of Pollution Peter Russ and Lindsay Tanner 1978 Heavy Metals in Port Phillip Bay
"…In 1972, Swinburne Institute of Technology chemical engineering students carried out a study of cadmium and zinc levels in mussels found on the eastern side of the bay. The results were disturbing. This study is recorded in the recently compiled Conservation Ministry Inventory of Heavy Metals Research in Victoria which states: 'Levels of cadmium and zinc found in shellfish often exceeded the recommended safety limit - and this prompted Dr Culka (the study supervisor) to write to the Fisheries and Wildlife Division to inform them of this fact'.
Yet it was not until David Phillip's Herald article of 30 September 1975 was published that the EPA and Health Department began to investigate the entire situation. Phillip's results caused a sudden furore among ministry scientists, and served notice to the government that the situation was indeed serious.
Apart from the very high results for Corio Bay, Phillips also found that Mussels at Mordialloc were above the 2 ppm standard for cadmium, and that at least at two other sites cadmium levels in mussels were above 1.7 ppm. Phillip's results for lead in mussels were considerably more serious, revealing that lead contamination was much more widespread than cadmium pollution. Of Phillip's 20 sampling sites for which lead in mussels was analysed, ten showed lead concentrations above the accepted standard of 2 ppm. The highest level recorded was 10.02 ppm in mussels at Sandringham, with a level of 3.69 recorded at St.Kilda.
Phillip's claim that his cadmium levels were higher than anywhere else in the world at that time appears to have been correct. His study of comparative levels for lead in mussels indicates that his results for lead were also the highest yet recorded in the world. Not surprisingly, the confidential Fisheries and Wildlife report of October 1975 recommended an immediate Health Department investigation into high lead, cadmium and zinc levels recorded by Phillips. Soon after, Fisheries and Wildlife and the Health Department tested mussels in Port Phillip Bay to determine the accuracy of Phillip's results.
The results correlated well with Phillips, and in the case of the Health Department were considerably higher, probably because their analytical techniques were not as precise. Tests by the Fisheries and Wildlife Division laboratories and the Department of Agriculture Marine Chemistry Unit achieved results very close to those recorded by Phillips.
In a letter of 30 October 1975 written by Dr John Harris of the Department of Agriculture Marine Chemistry Unit to Dr Alistair Gilmour of the Marine Pollution Studies Group, Harris said:
"In view of the recent notoriety given to the level of trace metals in Port Phillip Bay mussels, the Marine Chemistry Unit undertook the analysis of a limited number of mussels from Port Phillip Bay. The results of our analysis of the first lot of samples agrees with the results of Mr Phillips. These values are very high and a research programme is needed to identify the sources of the metals and how they are incorporated into Port Phillip Bay organisms."
Dr Gilmour then wrote to the director of the Environmental Studies section of the ministry, Dr Tom Linton, on 11 November 1975, stating: 'In view of the high levels, particularly of lead, I suggest that there is an urgent need to assess the ecological implication of this data'.
These statements suggest strongly that government scientists agreed with Phillip's interpretation of the relative seriousness of the situation. Phillip's results for cadmium were also apparently confirmed by data contained in a document tabled by the Labor member for Brunswick West, Mr Tom Roper, during a no-confidence motion in Borthwick. This document was from a Fisheries and Wildlife file, and contained hand-written notes made by Marine Fisheries Head McCloskey. The notes mentioned a Health Department report on cadmium in shellfish completed in May 1973, which found very high levels in oysters and scallops in certain parts of the bay. McCloskey also apparently suggested that Victoria should adopt a cadmium standard for fish and shellfish of 0.6 or 0.7 ppm, which evoked the comment from Sanders that such a stringent standard would have serious consequences for the scallop fishing industry.
Phillips' results for cadmium and lead in Port Phillip Bay mussels were subsequently confirmed by Latrobe University researchers Victor Talbot, under the nominal supervision of former EPA Chief Water Quality Officer Dr Hussain. Talbot's results were published in the Marine Pollution Bulletin of May 1976, but they were not published in the press until late November.
Talbot's results for cadmium showed that the 2 ppm standard for cadmium was exceeded at 15 out of his 22 sampling sites around the bay, with three of those 15 being described as 'borderline'. Talbot's results for lead in mussels were even more serious. At 19 out of his 22 sampling sites the lead level in mussels exceeded the standard of 2 ppm. The interpretation of these results given by Talbot, Magee and Hussain in their preliminary paper was as follows:
"With the exception of seven locations, the results for mussel analysis of this metal (cadmium) indicate that the waters of Port Phillip Bay and Corio Bays in June 1975 could be regarded as heavily polluted. Even those locations not too polluted are, however, close to it. If, on the other hand, WHO standards and not NHMRC standards are used, the whole bay may be considered as being polluted.
This element (lead) is present in the bay at levels unacceptable by NHMRC standards for food. It appears to be even more widespread than cadmium, although to be less toxic."
At around the same time, Talbot also conducted a study of heavy metals levels in Port Phillip Bay sediments, which was published in the Marine Pollution Bulletin in March 1976. His study again showed significant cadmium and lead pollution in most parts of the bay.
The EPA heavy metals-in-sediments survey of December 1975/January 1976 and May 1976 revealed that, apart from Corio Bay, cadmium levels in sediments were significantly high in a number of areas in the bay, the highest other level being 23 ppm at the outlet of Mordialloc Creek. Lead in sediments in some instanced reached enourmous levels, the highest recorded being 472 ppm near Cowderoy Street drain, 353 ppm at Koroit Creek, 300 ppm at Mordialloc Creek, 252 ppm at Fisherman's Gully drain, and 213 ppm at Kananook Creek. These levels were much higher than those recorded by Talbot, possibly because the sampling sites were different and the EPA analytical techniques were inadequate. Two levels for mercury in sediments were significantly higher than the remaining parts of the bay. Corio Bay recorded 1.8ppm and Mordialloc Creek 0.4 ppm mercury in sediments. One level, that taken near the outlet of Kororoit Creek, was an extremely high 21 ppm. The EPA survey also analysed copper and zinc levels in sediments, the highest results for which were 793 ppm copper at Mordialloc Creek, and 133 zinc at the same site…"
Port Phillip Bay Environmental Study
Impact of Shipping and Dredging on Toxicants in Port Phillip Bay
G.J. Fabris, C.A. Monahan, G.F. Werner and T. Theodoropoulos
(Victorian Fisheries Research Institute, Queenscliff, 3225.
CSIRO Australia, Technical Report No. 20, September 1995.
Selected Quotes
Summary
" . . . Sediments in the Port of Melbourne contain elevated concentrations of copper, nickel, lead, zinc, mercury and petroleum hydrocarbons compared to both Bay-wide sediments and to sediment quality guidelines published in scientific literature. Concentrations of butyltin compounds are generally low, however some sediment samples contained very high concentrations. Dumping of these sediments on the spoil ground located in the northern part of the Bay has resulted in elevated concentrations of metals and petroleum hydrocarbons in the spoil ground sediments. The concentrations of nickel and mercury in parts of the spoil ground are such that they could cause adverse biological effects to biota inhabiting the sediments. It is unlikely that leaching of dissolved toxicants from the sediments into the water column during dredging operations is significant. The annual loads of metals such as cadmium, copper, lead and mercury transported from the Port of Melbourne to the spoil ground is less than the annual load that enters the Bay from rivers, streams and drains. . ."
p1 "1.2 Dredging
Maintenance dredging to remove accumulated sediments is periodically carried out in dock areas and along shipping channels in Port Phillip Bay. Maintenance dredging by the Port of Melbourne and Port of Geelong authorities involves the dredging and subsequent dumping of more than 500,000 m3 of material annually (Batley 1992). Capital works dredging programs create additional volumes of sediment that are translocated from one part of the Bay and deposited on spoil grounds in another part of the Bay. Dredged sediments - particularly those from Port areas - may contain elevated concentrations of heavy metals, petroleum hydrocarbons, polynuclear aromatic hydrocarbons (PAH) and antifouling paint constituents. During dredging and dumping operations there is the potential for a proportion of these contaminants to be remobilised into the water column. Transport of substances released into the water column can adversely affect biota located in areas away from the dredging and spoil ground sites. . ."
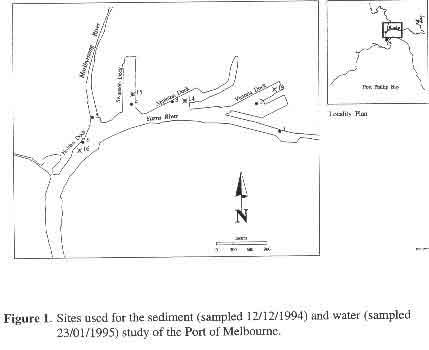
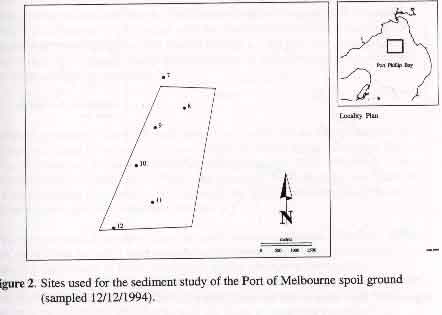
p8 3.1.2 Metals and Organics Results
". . . In the Port of Melbourne cores the concentrations of cadmium, chromium, copper, nickel, lead, zinc and mercury are consistently higher than the concentrations of the corresponding elements in cores collected from relatively uncontaminated areas of the Bay during 1993 (Fabris et al. 1994). Additionally, unlike the Bay core samples in which concentrations of copper, lead and zinc decreased with increasing depth, the metal and organics concentrations within the Port of Melbourne core segments did not change appreciably with depth. Dredging in the Port of Melbourne is carried out using a cutter-suction dredge that typically removes a 30 cm thick layer of material. Therefore the material that is deposited at the spoil ground would consist mostly of material contaminated with metals and organics having concentrations greater than those that occur in Bay sediments generally..."
p10 " *spoil from Port of Melbourne dredging operations has been dumped predominantly in the northern half of the spoil grounds, and;
*The surface (0-40cm) sediments of the spoil ground as a whole have progressively become more contaminated with cadmium, copper, lead, zinc and petroleum hydrocarbons compared to the surrounding Bay-wide sediments."
Table 2 (below). Comparison of toxicant concentrations in sediments from Port of Melbourne, spoil ground north and spoil ground south using Duncan's multiple range test (alpha=0.05 and confidence=0.95). The Type III SS general linear model significance level P is indicated. Tests were performed on natural logarithm transformed data. Values in the Table are arithmetic squares means. Data are ug g-1 dry weight (Fe is %).
| Variable | Port of Melbourne a | Spoil Ground North a | Spoil ground South a | P (d.f.=2) | Sediment Quality ERL b | Sediment Quality ERM b |
| Cd | 0.57 A | 0.44 A | 0.20 B | 0.0015 | 1.2 | 9.6 |
| Cr | 34.8 A | 30.3 AB | 28.1 B | 0.032 | 81 | 370 |
| Cu | 48.5 A | 34.3 B | 26.1 B | 0.0001 | 34 | 270 |
| Fe | 1.97 A | 1.96 A | 1.96 A | 0.9840 | nd | nd |
| Mn | 73.8 A | 59.3 B | 51.8 B | 0.0002 | nd | nd |
| Ni | 28.8 A | 23.7 B | 22.0 B | 0.0067 | 20.9 | 51.6 |
| Pb | 89.7 A | 74.2 A | 43.2 B | 0.0122 | 46.7 | 218 |
| Zn | 246 A | 194 A | 113 B | 0.0005 | 150 | 410 |
| Hg | 0.46 A | 0.24 B | 0.32 AB | 0.0574 | 0.15 | 0.71 |
| TPH | 546 A | 282 B | 150 C | 0.0002 | nd | nd |
| TBT c | 0.647 A | 0.0053 B | 0.0043 B | 0.0207 | nd | nd |
| DBT | 0.0257 A | 0.0081 A | 0.0043 A | 0.1138 | nd | nd |
| MBT | 0.0103 A | 0.0065 A | 0.00103 A | 0.7689 | nd | nd |
| Total PAH | 1.26 A | 0.92 A | 0.67 A | 0.2432 | 1.70 d | 9.60d |
a. for each variable, areas with the same superscript letters are not significantly different from each other. Common superscripts (e.g. AB) indicate that the mean at that area was not different to the means of either of the other two areas.
b. Long et al. (1995). ERL - Effects Range Low designated the lower 10th percentile of biological effects data. ERM - Effects Range Median designated by the 50th percentile of effects of biological effects data.
c. mean TBT for Port of Melbourne is 0.032 ug g-1 if two high values from Swanson Dock core (Appendix 3) are excluded
d. high molecular weight PAH (as determined in this study).
nd. no data
p11 "... The sediments from the Port of Melbourne area contained concentrations of copper, nickel, lead, zinc and mercury that exceed the 10th percentile of biological effects data (ERL, Table 2). The sites from the northern half of the spoil ground contained levels of nickel, lead, zinc and mercury that exceed ERL data. Nickel and mercury from all three areas exceed the ERL data. However, none of the areas exceed the 50th percentile of biological effects data (ERM, Table 2)..."
p13 "... The top 20 cm of sediment from the Swanson Dock (Site 4, Figure 1) and from the spoil ground (Site 7, Figure 2) were among the most contaminated in terms of metal petroleum hydrocarbons and butyltin compounds... To asses whether any of the contaminants present in these sediments could be readily leached into the water column during dredging and disposal operations the sediments were subjected to an elutriation test as specified in the Victorian Trial Dredge Protocol (EPA 1992, Schedule A). The weight to weight ratio of water sediment specified by the Protocol is 4:1. The metals results for the Swanson Dock sample ... were comparable to Bay-wide concentrations of dissolved metal species (Fabris and Monahan 1995). The butyltin data were also very low, and the TBT concentration was below the detection limit. The elutriate from the spoil ground site had higher concentrations of most of the metals, TPH and butyltin compunds than the Swanson Dock sample. The high iron concentration in this sample suggests that the elevated concentrations in this elutriate sample might be associated with fine suspended matter that passed through the 1 um pore size glass fiber filters used to filter samples."
p14 "Overall, the results of the elutriation test carried out during this study were lower than previously reported for samples from the Port of Melbourne area... On the basis of the results from the present study (and taking into account the dilution factors that are involved) it is unlikely that any dissolved metal, TPH and TBT species that might be released into the water column during dredging or disposal of the sediments at the spoil ground will significantly alter existing concentrations of dissolved species of these substances in the water column.
However, since the sediments dredged from the Port of Melbourne consist primarily of fine material, it is likely that contaminated suspended matter resulting from the dredging and spoil dumping operations could remain in the water column (as evidenced by the results of the elutriation test of the spoil ground sediment) long enough to be transported further afield than the immediate dredging or dumping area..."
p14 3.1.3 Toxicant Loads in Dredged Material
3.1.3.1 Port of Melbourne
"On average, maintenance dredging in the Port of Melbourne removes about 200,000 m3 to 250,000 m3 of material annually. Additional material may be occasionally dredged because of capital works programs (Captain Tim Muir, PMA, pers. comm). Table 6 shows that between 1987 and 1994 an annual average of 335,063 m3 of material was dredged from the Port of Melbourne. All of this material was deposited in the Port Phillip spoil ground (Figure 2). Most of the dredging (78%) during this period was carried out in areas north of the Westgate Bridge. All of the sediment samples for the Port of Melbourne study were collected north of the Westgate Bridge."
Table 6. Summary data for dredging operations in the Yarra River area of the Port of Melbourne for the period September 1987 to November 1994 a.
| Year | Volume dredged m3 (whole port) | Volume dredged m3 (north of Westgate Bridge) | Volume dredged m3 (south of Westgate Bridge) |
| 1987 | 131470 | 44621 | 86849 |
| 1988 | 757777 | 569073 | 188704 |
| 1989 | 394722 | 323967 | 70755 |
| 1990 | 445500 | 397200 | 48300 |
| 1991 | 475633 | 464083 | 11500 |
| 1992 | 143400 | 130300 | 13100 |
| 1993 | 24900 | 23300 | 1600 |
| 1994 | 307104 | 150055 | 157049 |
| Annual mean | 335063 | 262825 | 72238 |
| % of total | 78 | 22 |
p15 "Measurements on three samples indicated that the sediments from the Port of Melbourne contain 540kg of dry material per cubic metre of wet sediment. This value, together with the data from Tables 3 and 6, was used to calculate the annual loads of toxic substances that are transported from the port to the spoil ground as a result of dredging activities (Table 7).
Comparison of the mean annual metal loads contained in sediments dredged from the Port of Melbourne with loads contained in an equivalent volume of Bay-wide sediments shows that cadmium, copper, lead and mercury loads in the Port of Melbourne sediments exceed those in Bay-wide sediments by a factor of three or more (Table 7). TPH loads in the Port of Melbourne sediments are about 21 times greater than the load in comparable volumes of Bay-wide sediments (Table 7). To place these results in perspective, available data indicates that the mean annual metal loads contained in the dredged sediments from the Port of Melbourne (excepting Manganese) are lower than the annual loads of metals that enter the Bay from streams, drains and the Western Treatment Plant (Table 7). Input streams loads for petroleum hydrocarbons are not available at the time of writing, but reported spills (from ships) in the whole of Port Phillip Bay amount to approximately 15 tonnes of oil annually (D.Palmer 1995, Victorian Marine Pollution Committee; pers . comm.)..."
3.1.3.2 Port of Geelong
p 15/16 "Maintenance dredging of the shipping channels within the Inner Harbour (Corio Bay) and Outer Harbour (Geelong Arm) has been carried out periodically since the shipping channels were created circa 1854. Cutting of the channels and their maintenance since then has involved the dredging and disposal of about 20 million m3 of material (PGA 1993a). Additional proposed works to increase the depth of the shipping channels from 11m to 12 m will involved the dredging and disposal of about 2.1 million m3 of material from the Inner Harbour and about 3 million m3 of material from the Outer Harbour (Table 8). It is intended that sediments dredged from the Inner Harbour will be disposed of at a new spoil ground within the Inner Harbour and spoil from the Outer Harbour will be disposed of at a new spoil ground located in the Outer Harbour."
Table 7. Estimates of toxicant loads contained in sediments that are dredged or proposed to be dredged from the Ports of Melbourne (annual mean) and Geelong (channel improvement) compared to toxicant loads in equivalent volumes of Bay sediments and estimated annual loads entering the Bay from streams. Units in tonnes.
| Toxicant | PMA a | Bay-wide a (PMA volume) | Port of Geelong (inner channel) | Port of Geelong (inner spoil) | Port of Geelong (outer channel) |
Port of Geelong (outer spoil) |
Load from stream inputs (incl. WTP) c |
| Cd | 0.1 | 0.03 | 0.15 | 0.41 | 0.08 | 0.08 | 0.33 |
| Cr | 6.3 | 7.5 | 51.1 | 35.7 | 42.0 | 35.6 | 16.5 |
| Cu | 8.8 | 1.3 | 10.9 | 4.2 | 14.3 | 4.2 | 17.3 |
| Fe | 3564 | 5609 | 37095 | 29090 | 44105 | 27116 | 3788 |
| Mn | 13.4 | 15.7 | 261.4 | 95.4 | 249.2 | 103.1 | nd |
| Ni | 5.2 | 4.2 | 58.5 | 44.3 | 42.1 | 24.5 | 14.1 |
| Pb | 16.2 | 2.9 | 34.4 | 46.0 d | 23.9 | 19.4 | 31.1 |
| Zn | 44.5 | 19.3 | 42.8 | 45.0 | 36.8 | 36.2 | 126.8 |
| Hg | 0.08 | 0.02 | 0.1 | 0.09 | 0.07 | 0.03 | 0.1 |
| TPH | 95 | 4.7 | 56.5 | 1.2 | 20.3 | 4.2 | nd |
| PAH | 0.23 | 0.14 | 0.13 | 0.20 | 0.08 | 0.07 | nd |
| TBT | 0.006 | nd | 0.0005 | 0.0001 | 0.003 | 0.0002 | nd |
nd=no data
a. calculated from data in Tables 3 and 6 and assuming 540 kg of dry material per m3 of wet sediment.
b. calculated from data in Tables 3 and 8 and assuming 540 kg of dry material per m3 of wet sediment.
c. from Table 12 in Fabris and Monahan (1995).
d. 83 tonnes if the high lead concentration (note d, Table 3) is used.
Table 8 Summary data for the proposed dredging operations for the channel improvement in the Port of Geelong a.
| Volume dredged m3 (whole Port) | Volume dredged m3 (Inner Harbour) | Volume dredged m3 (Outer Harbour) |
| 5113000 | 2088000 | 3025000 |
p17 "The mean concentrations of toxic substances in the sediments of the Inner Harbour channels are generally higher than their respective concentrations in sediments from the Outer Harbour channels (Table 3). Industrial contamination of the Inner Harbour (Phillips et al. 1992) accounts for this difference. Similarly, the spoil ground sediments from the Inner Harbour are more contaminated than spoil ground sediments from the Outer Harbour for all variables measured except TPH and TBT (Table 3). TPH concentrations in the channel sediments are greater than those of the respective spoil grounds (45 times in the case of the Inner Harbour and 5 times in the case of the Outer Harbour; Table 3). The respective loads of metals and petroleum hydrocarbons are affected similarly. The petroleum hydrocarbon loads transported to the spoil grounds will therefore increase concentrations of petroleum hydrocarbons in the spoil grounds, with the Inner Harbour spoil ground being more affected than the Outer Harbour spoil ground..."
Aldrin is an organochlorine insecticide which is oxidized in the insect to form dieldrin, a neurotoxin. Aldrin was formerly used to kill insects such as termites and grasshoppers to protect crops such as corn and potatoes. It has been classified as a persistent organic pollutant. Due to health concerns regarding dieldrin, it is no longer manufactured or used in the United States. In addition, aldrin is itself a carconogen and mutagen.
Antimony is a chemical element in the periodic table that has the symbol Sb (Latin: stibium, meaning "mark") and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metalloid. Yellow and black antimony are unstable non-metals. Antimony is used in flame-proofing, paints, ceramics, enamels, a wide variety of alloys, electronics, and rubber.
Antimony and many of its compounds are toxic. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness, and depression. Larger doses cause violent and frequent vomiting, and will lead to death in a few days.
A study found that antimony is leaching from PET bottles (reported for some acidic fruit drinks), but at levels below drinking water guidelines. The guidelines are: *WHO 20ug/l. *US EPA, Health Canada and the Ontario Ministry of Environment, 6ug/l. *German Federal Ministry of Environment 5ug/l. *Japan 15ug/l.
The acidic nature of the drink is sufficient to dissolve small amounts of antimony oxide contained in the package of the drink; modern manufacturing methods prevent this occurrence. However, researchers are concerned that antimony levels correspond to duration the bottle is left to stand - the longer the water has been bottled, the higher the antimony leached.
Arsenic is very similar chemically to its predecessor, phosphorus. Similar to phosphorus, it forms colourless crystalline oxides As2O3 and As2O5 that are hygroscopic and readily soluble in water to form acidic solutions. Arsenic (V) acid, like phosphoric acid, is a strong acid. Like phosphorus, arsenic forms an unstable, gaseous hydride: arsine (AsH3). The similarity is so great that arsenic will partly substitute for phosphorus in biochemical reactions and is thus poisonous. However, in subtoxic doses, soluble arsenic compounds act as stimulants, and were once popular in small doses as medicinals by people in the mid 18th century. Lead hydrogen arsenate has been used, well into the 20th century, as an insecticide on fruit trees (sometimes resulting in brain damage to those working the sprayers), and Scheele's Green (a copper arsenate) has even been recorded in the 19th century as a colouring agent in sweets. In the last half century, monosodium methyl arsenate (MSMA), a less toxic organic form of arsenic, has replaced lead arsenate's role in agriculture.
Copper acetoarsenite was used as a green pigment known under many different names, including 'Paris Green' and 'Emerald Green'. It caused numerous arsenic poisonings. Arsenic poisoning kills by allosteric inhibition of essential metabolic enzymes, leading to death from multi-system organ failure.
The LD50 for pure arsenic is 763 mg/kg (by ingestion) and 13mg/kg (by intraperitoneal injection). For a 70 kg (~155lb) human, this works out to about 53 grams (less than two ounces). However, compounds, containing arsenic can be significantly more toxic. Chronic arsenic poisoning results from drinking water with high levels of arsenic over a long period of time. This may occur due to Arsenic contamination of groundwater.
Effects include changes in skin colour, formation of hard patches on the skin, skin cancer, lung cancer, cancer of the kidney and bladder, and can lead to gangrene. The World Health Organisation recommends a limit of 0.01mg/L of arsenic in drinking water; consumption of higher levels over long periods of time can lead to arsenicosis. Non-carcinogenic chronic effects include liver injury - jaundice and cirrhosis, peripheral vascular disease involving blueness of the extremeties, Raynaud's syndrome, and blackfoot disease (a type of gangrene), anemia, resulting from impaired heme biosynthesis, hyperkeratosis of the skin. There are also multiple lines of evidence for the carcinogenic effects of arsenic.
Arsenic has been known to cause many problems in third world countries where ground water supplies have been contaminated by arsenic derived from geologically recent fluvial deposits containing arseno-pyrites. This is a particular problem in Bangladesh where tube wells installed since the 1970s have intercepted ground waters flowing in fluvial deposits. Concentrations in these wells can exceed 1 part per thousand whereas the WHO maximum level is 10 parts per billion.
It has also been confirmed that natural arsenic contamination of drinking water has also been a problem in wells in New Hampshire, USA. Chronic low level arsenic poisoning, or arsenicosis, such as is seen in Bangladesh can potentially result in the victim developing cancer.
Barium is a chemical element. It has the symbol Ba, and atomic number 56. Barium is a soft silvery metallic alkaline eath metal. It is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with water and carbon dioxide and is not found as a mineral.
Barium compounds are extremely poisonous. At low doses, barium acts as a muscle stimulant, while higher doses affect the nervous system, causing cardiac irregularities, tremors, weakness, anxiety, dyspnea and paralysis. This may be due to its ability to block potassium ion channels which are critical to the proper function of the nervous system. Unlike other heavy metals, barium does not bioacculmulate. However, inhaled barium dust can accumulate in the lungs, a benign condition called baritosis.
Beryllium is the chemical element that has the symbol Be and atomic number 4. A bivalent element, elemental beryllium is a steel grey, strong, light-weight yet brittle, alkaline earth metal. It is primarily used as a hardening agent in alloys (most notably beryllium copper). Beryllium and its salts are toxic substances and potentially carcinogenic. Chronic berylliosis is a pulmonary and systematic granulomatous disease caused by exposure to beryllium. Acute beryllium disease in the form of chemical pneumonitis was reported in Europe in 1933 and in the United States in 1943. Cases of chronic berylliosis were first described in 1946 among workers in plants manufacturing fluorescent lamps in Massachusetts. Chronic berylliosis resembles sarcoidosis in many respects, and the differential diagnosis is often difficult.
Some people (1-15%) become sensitive to beryllium. These individuals may develop an inflammatory reaction that principally targets the respiratory system and skin. This condition is called chronic beryllium disease (CBD), and can occur within a few months or many years after exposure to higher than normal levels of beryllium (greater than 0.02ug/m3). This disease causes fatigue, weakness, night sweats and can cause difficulty in breathing and a persistent dry cough. It can result in anorexia, weight loss, and may also lead to right-side heart enlargement and heart disease in advanced cases. Some people who are sensitised to beryllium may not have any symptoms.
There are no studies on the health effects of children exposed to beryllium, although individual cases of CBD have been reported in children of beryllium workers from the 1940s. It is likely that the health effects seen in children exposed to beryllium will be similar to the effects seen in adults. It is unknown whether children differ from adults in their susceptibility to beryllium. It is unclear whether beryllium is teratogenic.
Compliance with the current U.S. Occupational Safety and Health Adminstration (OSHA) permissable exposure limits for beryllium of 2 ug/m3 has been determined to be inadequate to protect workers from developing beryllium sensitisation and CBD. The American Conference of Government Industrial Hygienists (ACGIH), which is an independent organisation of experts in the field of occupational health, has proposed a threshold limit value of (TLV) of 0.05ug/m3 in a 2006 Notice of Intended Change (NIC). This TLV is 40 times lower than the current OHSA permissable exposure limit, reflecting the ACGIH analysis of best available peer-reviewed research data concerning how little airborne beryllium is required to cause sensitisation and CBD.
Cadmium is a chemical element in the periodic table that has the symbol Cd and atomic number 48. A relatively rare, soft, bluish-white, transition metal, cadmium is known to cause cancer and occurs with zinc ores. Cadmium is used largely in batteries and pigments, for example in plastic products. Cadmium is an occupational hazard associated with industrial processes such as metal plating and the production of nickel-cadmium batteries, pigments, plastics and other synthetics. The primary route of exposure in industrial settings is inhalation. Inhalation of cadmium-containing fumes can result initially in metal fume fever but may progress to chemical pneumonitis, pulmonary oedema, and death.
Cadmium is also a potential environmental hazard. Human exposures to environmental cadmium are primarily the result of burning of fossil fuels and municipal wastes. However, there have been notable instances of toxicity as the result of long-term exposure to cadmium in contaminated food and water. In the decades following World War II, Japanese mining operations contaminated the Jinzu River with cadmium and traces of other toxic metals. Consequently, cadmium accumulated in the rice crops growing along the riverbanks downstream of the mines. The local agricultural communities consuming the contaminated rice developed Itai-itai disease and renal abnormalities, including proteinuria and glucosuria.
Cadmium has been reported by many workers to be harmful to all living systems with no known biological requirement. Although work by Von Zglinicki et al (1992) has postulated that very low levels of cadmium may stimulate DNA synthesis and cell growth. A relationship between the chemistry and the toxicity of cadmium was shown by Bienvenu et al., (1963) when he determined the dose of various soluble metal compounds given to mice that resulted in the death of 50% of the animals within 30 days (LD50). He noted regularities between the relative toxicity and the position of the element in the periodic table.
The stongest trend appears to be the general decrease in the LD50 in the order: M+ ions of the group Ia, M2+ ions of group IIa, M3+ ions of the group IIIa, M2+ ions of the first transition series and then M+, M2+ and M3+ ions of the post transition element groups Ib - IIIb. A second trend appears in the M2+ transition metal cations, this is a decrease in the LD50 with increasing atomic number. The greater toxicity of the heavy metals is also apparent within groups IIb and IIIb. The graph highlights cadmium, a divalent, post transition metal, as one of the most toxic elements with a LD50 of 0.000033g-moles/kg. Only indium (as In3+) and mercury (as Hg2+ were found to be more toxic.
All heavy metals can form a wide variety of coordination compunds and ions that bind to various polydentate organic ligands. The binding of such metal ions appears to link enzymes to substrates and may be partly responsible for the power of enzymes to catalyst biochemical recations. The most important donor atoms that link metal ions to biological molecules are oxygen (O), nitrogen (N) and sulphur (S). This can be shown as a series of bidentate ligands which are attached to the metal ion by two donor atoms to form a chelate complex: oxalate (OO), glycine (ON), ethylene diamine (NN), mercaptyl acetate (SO) and mercapty amine (SN).
It can be shown that cadmium should not displace any of the metals shown from an oxygen donating ligand but will displace Mn2+ and Fe2+ from nitrogen donors. Cadmium is bound to sulfur more strongly that all the metals shown except CU2+ and Hg2+. The very extended capability of cadmium to bind nucleophillic sites of macromolecules (proteins, DNA and RNA) accounts for the multiplicity of toxic effects observed in vitro and in vivo. These chemical reactions and the relatively strong bonding of cadmium to sulphur donating enzymes may partly explain the effect of small amounts of cadmium on living systems.
Long-term exposure to cadmium results in an irreversible tubular nephropathy that may develop into renal insufficiency. The cadmium-metallothionein complex is taken up almost quantitatively from the glomerular filtrate by epithelial cells of the proximal tubule and rapidly degraded by lysosomes. Although biosynthesis and metallothionein, in response to cadmium ions liberated by proteolysis, also occurs in these cells. It has been reported by a number of authors that part of the cadmium ions escapes this cytosolic binding system and reaches other subcellular targets (such as mitochondria and the nucleus). As a result, the reabsorption of low molecular weight proteins (including B2-microglobulin), ions (calcium and phosphate) and small solutes (glucose and aminoacids) is irreversibly impaired.
The relationship between the critical concentration of cadmium in renal cortex and the early signs of tubular dysfunction in humans has been the subject of numerous studies in both groups of exposed workers and populations living in polluted areas. A limit of urinary cadmium excretion of approximately 4 7g/g of creatinine has been derived from survesy conducted in the Ishikawa prefecture of Japan (Itai-Itai disease). Beyond this level the excretion of B2-metallothionein-cadmium complex becomes pathological.
Belgian studies have found evidence that the threshold of urinary cadmium for increased excretion of low molecular weight proteins, aminoacids and calcium is approximately 2 7g/g. This is reached in 10% of subjects in the general population and increases to 16% of the population over the age of 60. The retrospective study of the causes of mortality, from 1969 to 1976, indicates a very significant increase in mortality from nephrosis and nephritis in women over 60 and living in the Liege conurbation. This area has been markedly polluted by cadmium emissions from zinc smelters.
Although the Itai-Itai syndrome, in which renal insufficiency is associated with osteoprosis and osteomalacia, has been studied for more than twenty years, the precise pathogenic mechanism of this disease has not been fully established. It is not known whether bone demineralisation is secondary to nephrotoxic damage or results from a direct effect on bone tissue. Studies conducted in Japan strongly suggest that osteopathies and mortality, at least in women, are closely related to the kidney damage characterised by the urinary excretion of the B2-metallothionein-cadmium complex.
Three hypotheses have been formulated for osteotoxicity and partially confirmed by experiments on animals, tissue and cells in culture. The first involves an inhibition of the formulation of the dihydroxylated metabolite of vitamin D3 leading to a reduction in the incorporation of cadmium in the bone and ultimately to osteomalacia. The production of this active metabolite of vitamin D3 is dependant on cyclic AMP, adenylate cyclase, parathyroid hormone and cytochrome(s) P-450, all of which are factors adversely affected by exposure to cadmium. The second hypothesis implies that cadmium counteracts the absorption of calcium in the small intestine, decreasing the bioavailability of this element and producing a decalcification of bone that is the primary characteristic of osteoporosis. The third proposes a disturbance of collagen in bone by inhibition of lysyl-oxidase.
Nutritional deficiencies (calcium and vitamin D), multiparity and hormonal factors are key factors in the development of cadmium-induced bone pathology. It has been reported that cadmium causes bone lesions in rodents when associated with a hypocalcic diet but this is at a lesser magnitude than that found in Itai-Itai patients. In Japanese women with renal dysfunction that was associated with bone damage or not, the isozyme of alkaline phosphatase originating from bone tissue was elevated by cadmium exposure. This indicates a direct effect of cadmium on bone mineralisation.
Many studies, mostly carried out more than 15 years ago, have demonstrated that chronic oral administration of low levels of cadmium to rats, via food or drinking water, causes a rise of arterial pressure. These studies indicate that the pressor effect of cadmium can only be observed in the presence of a range of experimental conditions (strain, composition of the diet, exposure time, etc). None of these studies have shown the mechanism(s) of the hypertensive effect of cadmium.
This is not surprising as blood pressure is regulatred by many systems that may be mutually compenating. The pressor effect of cadmium may be regarded as a direct effect on vasculature, a functional damage to the kidneys and an altered liberation of neuromediators. Some surveys, on small groups of human with low-level environmental exposure, have found a positive association between the accumulation of cadmium and an increase in blood pressure. Other surveys have failed to confirm it or even have revealed negative correlations between the two variables.
None of the studies conducted in several cadmium-polluted districts in Japan, the United Kingdom, Belgium of in the United States have shown evidence of a positive relationship between blood pressure, the prevalence of cardiovascular diseases and urinary cadmium. Also, the significant increase in mortality in Japanese suffering from tubular proteinuria is not associated with a greater prevalence of hypertension and cardiovascular disease.
In rodents, acute intratesticular administration has long been shown to produce vascular lesions and interstitial oedema provoking a reduction in the produstion of androgens and a failure of spermiation. However, the effect of low-level expose on male reproductive organs is less obvious. Data relating to man is very limited and indicates that moderate long-term exposure has little effect on male sterility. Pre and post natal exposure of female rats to cadmium produced a reduction of oestrogen production and development of the utero-ovarian function. This function is also affected in adult females, particularly with regard to the implantation and growth of the embryo.
Exposure to cadmium during successive pregnancies incresaes the amount of metal stored in the kidneys of mice and it has been suggested that in mammals, including man, maternal exposure produces a placental accumulation of the metal and a subsequent loss of zinc. He suggested that the decrease in placental Zn/Cd ration observed in mothers that smoke tobacco, may be associated with a reduced foetal growth and consequently a decreased birth weight.
Cadmium appears to be both a direct and indirect mutagenic agent in both bacteria and mammalian cells. The most frequent chromosomic aberrations in eucaryote cells are ruptures and lacunae (gaps) rather than exchanges. Aneuploidy and blockade of meiotic division have also been found in yeasts and oocytes, demonstrating that cadmium acts as a cytoskeletal poison. Studies of lymphocyte chromosones from human subjects exposed to cadmium in the workplace or suffering from Itai-Itai have produced conflicting results.
Epidemiological data on groups of exposed workers in the USA and Europe has been collected from the beginning of 1960 and updated periodically. This shows a very clear relationship between long-term exposure to cadmium oxide dust and fumes and the prevalence of cancers of the respiratory tract and a weaker relationship to prostate cancers. Cadmium has been placed in group I of the IARC classification of carcinogens. No clear relationship between the prevalence of internal cancers and the dietary intake of cadmium has been shown in countries such as Japan and Belgium where environmental contamination is high. Limited data from Canada and the USA suggest that there may be a positive link between the overall level of environmental pollution by cadmium and prostate cancer.
Chlordane is a manufactured chemical that was used as a pesticide in the United States from 1948 to 1988. It does not occur naturally in the environment. It was sold by Chevron as a white powdery dust. When mixed with water it becomes a colourless to amber thick liquid. Until 1983, chlordane was used as a pesticide on crops like corn and citrus and on home lawns and gardens. Chevron specifically marketed it as an ant and termite killer.
Because of concern about damage to the environment and harm to human health, the Environmental Protection Agency (EPA) banned all uses of chlordane in 1983 except to control termites. In 1988, the EPA banned all uses of chlordane. The EPA recommends that a child should not drink water with more than 60 parts of chlordane per billion parts of drinking water (60ppb) for longer than 1 day. EPA has set a limit in drinking water of 2ppb.
Chlordane sticks strongly to soil particles at the surface and it is not likely to enter groundwater and so as a result it can stay in the soil for over 20 years and breaks down very slowly. Chlordane does not dissolve easily in water. It affects animal species because it builds up in the tissues of fish, birds, and mammals.
Chlordane affects the nervous system, the digestive system, and the liver in people and animals. Headaches, irritability, confusion, weakness, vision problems, vomiting, stomach cramps, diarrhea, and jaundice have occurred in people who breathed air containing high concentrations of chlordane or accidently swallowed small amounts of chlordane. Large amounts of chlordane taken by mouth can cause convulsions and death in people.
According to the ATSDR, a man who had long-term skin contact with soil containing high levels of chlordane had convulsions. Japanese workers who used chlordane over a long period of time had minor changes in liver function. Animals given high levels of chlordane by mouth for short periods died or had convulsions. Long-term exposure caused harmful effects in the liver of test animals. It is not known if chlordane affects the ability of people to have children or whether it causes birth defects. Animals exposed before birth or while nursing developed behavioural effects later.
Trivalent chromium (Cr(III), or Cr3+) is required in trace amounts for sugar metabolism in humans (Glucose Tolerance Factor) and its deficiency may cause a disease called chromium deficiency. In contrast, hexavalent chromium is very toxic and mutagenic when inhaled. Cr (VI) has not been established as a carcinogen when not inhaled but in solution its is well established as a cause of allergic contact dermatitis (ACD). Recently it was shown, that the popular dietary supplement chromium picolinate complex generates chromosone damage in hamster cells. In the United States, the dietary guidelines for daily chromium uptake were lowered from 50-200 ug for an adult to 35 ug (adult male) and to 25 ug (adult female).
Chromium metal and chromium (III) compounds are not usually considered health hazards, but hexavalent chromium (chromium VI) compounds can be toxic if orally ingested or inhaled. The lethal dose of poisonous chromium (VI) compounds is about one half teaspoon of material. Most chromium (VI) compounds are irriating to eyes, skin and mucous membranes. Chronic exposure to chromium (VI) compounds can cause permanent eye injury, unless properly treated. Chromium (VI) is an established human carcinogen. World Health Organisation recommended maximum allowable concentration in drinking water for chromium (VI) is 0.05 milligrams per litre. Hexavalent chromium is also one of the substances whose use is restricted by the European Restriction of Hazardous Substances Directive. Hexavalent chromium in contact with skin acts as both sensitiser and irritant. After entering the organism, its gets reduced to trivalent chromium, which then binds to proteins and creates haptens which trigger immune system reaction. Once developed, chrome sensitivity becomes fairly persistent; in such cases, even contact with chromate-dyed textiles or wearing chromate-tanned leather shoes can cause or exacerbate contact dermatitis.
In an organism, hexavalent chromium undergoes reduction, first to metastable pentavalent chromium, then to trivalent chromium. Pentavalent chromium is a known carcinogen. If the material gets lodged in tissues (lungs are especially vulnerable here, followed by fine capillaries in kidneys and intestines), its long-term action may lead to cancerous growth. In some parts of Russia, pentavalent chromium was reported as one of the factors of incidence of permature senility. The OSHA PEL for airborne exposures to hexavalent chromium is 5ug/m3 (0.005mg/m3).
Researchers have recently reported discovering that vitamin C reacts inside human lung cells with chromium 6, causing massive DNA damage. Low doses of chromium 6, combined with vitamin C, produce up to 15 times as many chromosomal breaks and up to 10 times more mutations, compared with cells lacking vitamin C. Outside cells, vitamin C actually protects against cellular damage caused by hexavalent chromium.
As chromium compounds used in dyes and paints and the tanning of leather, these compounds are often found in soil and groundwater at abondoned industrial sites, now needing environmental cleanup and remediation per the treatment of brownfield land. Primer paint containing hexavalent chromium is still widely used for aerospace and automobile refinishing applications.
DDT or Dichloro-Diphenyl-Trichloroethane is the first modern pesticide and is one of the best known synthetic pesticides. It was developed early in World War II, and initially used with great effect to combat mosquitoes spreading malaria, typhus, and other insect-borne human diseases among both military and civilian populations, and as an agricultural insecticide. The Swiss chemist Paul Hermann Muller of Geigy Pharmaceutical in Switzerland was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods."
DDT is an organochlorine insecticide. It is highly hydrophobic, colourless, crystalline solid with a weak, chemical odour. It is nearly insoluble in water but has a good solubility in most organic solvents, fats and oils. It is moderately toxic, with a rat LD50 of 113 mg/kg. DDT has potent insecticidal properties; it kills by opening sodium ion channels in insect neurons, causing the neuron to fire spontaneously. This leads to spasms and eventual death. Insects with certain mutations in their sodium channel gene may be resistant to DDT and other similar insecticides. DDT resistance is also conferred by up-regulation of genes expressing cytochrome P450 in some insect species.
DDT is a persistent organic pollutant with a half life of between 2-15 years, and is immobile in most soils. Its half life is 56 days in lake water and approximately 28 days in river water. Routes of loss and degradation include runoff, volatilization, photolysis and biodegradation (aerobic and anaerobic). These processes generally occur slowly. Breakdown products in the soil environment are DDE (1,1-dichloro-2,2-bis(p-dichlorodiphenyl)ethylene) and DDD (1,1-dichloro-2,2-bis(p-chlorophenyl)ethane), which are also highly persistent and have similar chemical and physical properties. These products together are known as total DDT.
DDT and its metabolic products DDE and DDD magnify through the food chain, with apex predators such as raptors having a higher concentration of the chemicals (stored mainly in body fat) than other animals sharing the same environment. In the United States, human blood and fat tissue samples collected in the early 1970s showed detectable levels in all samples. A later study of blood samples collected in the later half of the 1970s (after the US DDT ban) showed that blood levels were declining further, but DDT or metabolites were still seen in a very high proportion of the samples. Biomonitoring conducted by the CDC as recently as 2002 shows that more than half of subjects tested had detectable levels of DDT or metabolites in their blood, and of the 700+ milk samples tested by the USDA in 2005, 85% had detectable levels of DDE.
DDT is a toxicant across a range of phyla. In particular, DDT has been cited as a major reason for the decline of the bald eagle in the 1950s and 1960s as well as the peregrine falcon. DDT and its breakdown products are toxic to embryos and can disrupt calcium absorption thereby impairing egg-shell quality. Studies in the 1960s and 1970s failed to find a mechanism for the hypothesized thinning, however more recent studies in the 1990s and 2000s have laid the blame at the feet of DDE, but not all experts accept those claims. Some studies have shown that although DDE levels have fallen dramatically that eggshell thinness remains 10-12 percent thinner than pre=DDT thicknesses. In general, however, DDT in small quantities has very little effect on birds; its primary metabolite DDE, has a much greater effect. DDT is also highly toxic to aquatic life, including crayfish, daphnids, sea shrimp and many species of fish. DDT may be moderately toxic to some amphibian species, especially in the larval stages. In addition to acute toxic effects, DDT may bioaccumulate significantly in fish and other aquatic species, leading to long-term exposure to high concentrations.
The effects of DDT on human health are disputed since studies have yielded conflicting results.
*DDT is classified as "moderately toxic" by the US National Toxicological Program and "moderately hazardous" by WHO. It is not considered to be highly toxic, and in fact it has been applied directly to clothes or used in soap. Indeed, DDT has on rare occasions been administered orally as a treatment for barbituate poisoning.
*Occupational exposure to DDT was associated with reduced verbal attention, visumotor speed, sequencing, and with increased neuropsychological and psychiatric symptoms in a dose-response pattern (ie, per year of DDT application) in retired workers aged 55-70 years in Costa Rica. DDT or DDE concentrations were not determined in this study.
*In one 1969 study, 24 cynomolgus monkeys and rhesus monkeys fed 20mg/kg of DDT for 130 months were compared to a control group of 17 monkeys. The study demonstrated "clear evidence of hepatic and CNS toxicity following long-term DDT administration." Although the exposed group developed two malignancies and three benign tumours, compared to zero in the control group, statistically this is still "inconclusive with respect to a carcinogenic effect of DDT in nonhuman primates."
* In another study, humans voluntarily ingested 35 mg of DDT daily for about two years, and were then tracked for several years afterward. Although there was "suggestive evidence of adverse liver effects", no other adverse effects were observed.
*The EPA, in 1987, classified DDT as class B2, a probable human carcinogen based on "Observation of tumours (generally of the liver) in seven studies in various mouse strains and three studies in rats. DDT is structurally similar to other probable carcinogens, such as DDD and DDE". Regarding the Human Carcinogenicity Data, they stated "The existing epidemiological data are inadequate. Autopsy studies relating tissue levels of DDT to cancer incidence have yielded conflicting results. Three studies reported that tissue levels of DDT and DDE were higher in cancer victims than in those dying of other diseases. In other studies, no such relationship was seen. Studies of occupationally exposed workers and volunteers have been of insufficient duration to be useful in assessment of the carcinogenicity of DDT to humans.
*With detailed work history of chemical manufacturing workers to estimate DDT exposure, a nested case-control study reported occupational DDT exposure associated with increased pancreatic cancer risk. A weak association of self-reported DDT use with pancreatic cancer was reported in another case-control study. A report indicated a higher standardised mortality ratio for pancreatic cancer in outdoor workers with a history of DDT exposure of less than 3 years, but the standardised mortality ratio of DDT workers with exposure of 3 years or more was not significantly raised.
*A study examined 35 workers exposed to 600 times the average DDT exposure levels over a period of 9 to 19 years. No elevated cancer risk was observed.
The journal Cancer recently published a review of all of the epidemiological studies on breast cancer and DDT and DDE published between 2000 and 2006. The authors state that "Positive findings for well-controlled studies in the early 1990s of associations between breast cancer risk and the insecticide DDT, its breakdown product DDE, and PCBs prompted additional study. Snedeker reviewed studies of DDT/DDE and dieldrin, concluding that exisiting research strategies provided conflicting and mostly negative evidence...Updating the picture to 2006 provides...essentially unchanged conclusions for DDT/DDE." Turning their attention to the recent studies, they conclude that "A few studies show elevated risk," but "most studies did not support an association of DDE and breast cancer overall or stratified by menopausal status, tumour hormone receptor status, parity, breast-feeding, or body mass index...In light of these findings, additional study of incident breast cancer in association with biological measures of DDE/DDT levels near the time of diagnosis is not a promising avenue."
DDT, like many other organochlorines, has been shown to have weak xenoestrogenic activity; meaning it is chemically similar enough to estrogen to trigger hormonal responses in contaminated animals. This hormonal-mimicking activity has been observed when DDT is used in laboratory studies involving mice and rats as test subjects, and available epidemiological evidence indicates that these effects may be occurring in humans as a result of DDT exposure. A recent study conducted by the University of California, Berkeley suggests children who have been exposed to DDT while in the womb have a greater chance to experience development problems.
A review article in The Lancet concludes:
"Although DDT is generally not toxic to human beings and was banned mainly for ecological reasons, subsequent research has shown that exposure to DDT at amounts that would be needed in malaria control might cause preterm birth and early weaning, abrogating the benefit of reducing infant mortality from malaria. ... DDT might be useful in controlling malaria, but the evidence of its adverse effects on human health needs appropriate research on whether it achieves a favourable balance of risk versus benefit."
Acute oral LD50 for rats: 113-118, mice: 150-300, rabbits: 300, dogs: 500-750 and sheep and goats >1000 mg/kg. Acute percutaneous LD50 for female rats is 2510 mg/kg. The no observable effect level (NOEL) for rats is 1 mg/kg diet and 17 people who consumed 0.5 mg/kg body weight daily for 1.75 years suffered no ill effect. The Codex JMPR (2000) allowable daily intake (ADI) is 0.01mg/kg body weight and this is equivalent to 2 ug/L in water intake. DDT is moderately toxic to birds but is extremely toxic to fish and aquatic life. The LC50 (96 hour) for Coho salmon: 4, rainbow trout: 7 and yellow perch: 9ug/L. The LC50 (48 hour) for daphnia is 1.10ug/L and for Crangon septemspinosa (shrimp) is 0.4ug/L.
Dieldrin is a chlorinated hydrocarbon originally produced by Bayer AG as an insecticide. The molecule has a ring structure based on naphthalene. Dieldrin is closely related to aldrin which itself breaks down to form dieldrin. Aldrin is not toxic to insects, it is oxidised in the insect to form dieldrin that is the active compound. Both dieldrin and aldrin are named after the Diels-Alder reaction which is used to form aldrin from a mixture of norbornadiene and hexachlorocyclopentadiene.
Originally developed in the 1940s as an alternative to DDT, dieldrin proved to be a highly effective insecticide and was very widely used during the 1950s to early 1970s. Endrin is a stereoisomer of dieldrin. However, it is an extremely persistent organic pollutant, it does not easily break down. Furthermore it tends to accumulate as it is passed along the food chain. Acute (short-term) exposure is considered to be harmless, but long-term exposure has proven toxic to a very wide range of animals including humans, far greater than to the original insect targets. For this reason it is now banned in most of the world.
Dioxin is the common name for the group of compounds classified as polychlorinated dibenzodioxins (PCDDs). PCDDs, which are members of the family of halogenated organic compounds, have been shown to bioaccumulate in humans and wildlife due to their lipophilic properties, and are known teratogens, mutagens, and suspected human carcinogens. Dioxins are absorbed primarily through dietary intake of fat, as this is where they accumulate in animals and humans. In humans, the highly chlorinated dioxins are stored in fatty tissues and are neither readily metabolised nor excreted. The estimated elimination half-life for highly chlorinated dioxins (4-8 chlorine atoms) in humans ranges from 7.8 to 132 years.
The persistence of a particular dioxin congener in an animal is thought to be a consequence of its structure. It is believed that dioxins with few chlorines, which thus contain hydrogen atoms on adjacent pairs of carbons, can more readily be oxidised by cytochromes P450. The oxidised dioxins can then be more readily excreted rather than stored for a long time.
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic of the congeners. Other dioxin congeners (or mixture thereof) are given a toxicity rating from 0 to 1, where TCDD = 1. This toxicity rating is called the Toxic Equivalence Factor, or TEF. TEFs are consensus values and, because of the strong species dependence for toxicity, are listed seperate for mammals, fish and birds. TEFs for mammalian species are generally applicable to human risk calculations. The TEFs have been developed from detailed assessment of literature data to facilitate both risk assessment and regulatory control. Many other compounds may also have dioxin-like properties, particularly non-ortho PCBs, some of which have TEFs as high as 0.1.
The total dioxin toxic equivalence (TEQ) value expresses the toxicity as if the mixture were pure TCDD. The TEQ approach and current TEFs have been adopted internationally as the most appropriate way to estimate the potential health risks of mixture of dioxins. Recent data suggest that this type of linear scaling factor may not be the most appropriate treatment for complex mixtures of dioxins; further research into non-linear toxicity models is required to substantiate this hypothesis. Dioxins and other persistent organic pollutants (POPs) are subject to the Stockholm Convention. The treaty obliges signatories to take measures to eliminate where possible, and minimise where not possible to eliminate, all sources of dioxin.
Dioxins build up primarily in fatty tissues over time (bioaccumulate), so even small exposures may eventually reach dangerous levels. In 1994, EPA reported that dioxin is a probable carcinogen, but notes that non-cancer effects (reproduction and sexual development, immune system) may pose an even greater threat to human health. TCDD, the most toxic of the dibenzodioxins, has a half-life of approximately 8 years in humans, but at high concentrations, the elimination rate is enhanced by metabolism. The health effects of dioxins are mediated by their action on a cellular receptor, the aryl hydrocarbon receptor (AhR).
Dioxins also accumulate in food chains in a fashion similar to other chlorinated compounds (bioaccumulate). This means that even small concentrations in contaminated water can be concentrated up a food chain to dangerous levels due to the long biological hald life and low solubility of dioxins.
Exposure to high levels of dioxin in humans causes a severe form of persistent acne, known as chloracne. Other effects in humans may include:
*Developmental abnormalities in the enamel of children's teeth.
*Central and Peripheral Nervous System pathology.
*Thyroid disorders.
*Damage to the Immune systems.
*Endometriosis
*Diabetes.
While its has been difficult to prove that dioxins cause specific health effects in humans due to the lack of controlled dose experiments, studies in animals have shown that dioxin causes a wide variety of toxic effects. In particular, TCDD has been shown to be teratogenic, mutagenic, carcinogenic, immunotoxic, and hepatotoxic. Furthermore, alterations in multiple endocrine and growth factor systems have been reported. The most sensitive effects, observed in multiple species, appear to be developmental, including effects on the developing immune, nervous, and reproductive systems. These effects are caused at body burdens close to those reported in humans.
Among the animals for which TCDD toxicity has been studied, there is strong evidence for the following effects:
*Birth defects (teratogenicity) in rodents, including rats, mice, hamsters and guinea pigs, birds; and fish.
*Cancer (including neoplasms in the mammalian lung, oral/nasal cavities, thyroid and adrenal glands, and liver, squamous cell carcinoma, and various animal hepatocarcinomas) in rodents and fish.
*Hepatotoxicity (liver toxicity) in rodents, chickens and fish.
*Endocrine disruption in rodents and fish.
*Immunosuppression in rodents and fish.
*Learning.
Dioxin exposure incidents:
- In 1949 in herbicide production plant for 2,4,5-T in Nitro, West Virginia 240 people were affected when a relief valve opened.
- In 1963 a dioxin cloud escapes after an explosion in a Philips-Duphar plant (now Solvay Group) near Amsterdam. In the 1960s Philips-Duphar produced 2250 tonnes of 'Agent Orange' for the US Army.
- In 1976 large amounts of dioxin were released in an industrial accident as Seveso, although no immediate human fatalities or birth defects occurred.
- In 1978, dioxin was one of the contaminants that forced the evacuation of the Love Canal neighbourhood of Niagra Falls, New York. Dioxin also caused the 1983 evacuation of Times Beach Missouri.
- In the 1960s, parts of the Spolana chemical plant in Neratovice, Czechoslovakia, were heavily contaminated by dioxins, when the herbicide, 2,4,5-T (also a component of Agent Orange) was produced there. Workers in this factory were exposed to high concentrations of dioxins at that time. Dozens of them fell seriously ill. A possibly large amount of dioxins was flushed from the factory into the Labe River during the 2002 European flood. No direct consequences of this incident have thus far been recorded.
- From 1982 through to 1985, Times Beach, Missouri was bought out and evacuated under order of the U.S. Environmental Protection Agency due to high levels of dioxin in the soil. The town eventually disincorporated.
- In December 1991, an electrical explosion caused dioxin (created from the oxidation of Polychlorinated Biphenyl) to spread through four Residence Halls and two other buildings on the college campus of SUNY New Platz.
- In May 1999, there was a dioxin crisis in Belgium: quantities of dioxin had entered the food chain through contaminated animal feed. 7,000,000 chickens and 60,000 pigs had to be slaughtered. This scandal was followed by a landslide change in government in the elections one month later.
- On September 11, 2001 explosions released massive amounts of dust into the air. The air was measured for dioxin from September 23, 2001 to November 21, 2001 and reported to be "likely the highest ambient concentration that have ever been reported." [in history]. The EPA report dated October 2002 and released in December of 2002 titled "Exposure and Human Health Evaluation of Airbourne Pollution from the World Trade Centre Disaster" authored by the EPA Office of Research and Development in Washington stated that dioxin levels recorded at a monitoring station on Park Row near City Hall Park in New York between October 12 and 29, 2001 averaged 5.6 parts per trillion/per cubic metre of air, or nearly six times the highest dioxin level ever recorded in the U.S. Dioxin levels in the rubble of the World Trade Centers was much higher with concentrations ranging from 10 to 170 parts per trillion. The report did no measuring of the toxicity of indoor air.
- In a 2001 case study, physicians reported clinical changes in a 30 year old woman who had been exposed to a massive dosage (144,000 pg/g blood fat) of dioxin equal to 16,000 times the normal body level; the highest dose of dioxin ever recorded in a human. She suffered from chloracne, nausea, vomiting, epigastric pain, loss of appetite, leukocytosis, anemia, amenorrhoea and thrombocytopenia. However, other notable laboratory tests, such as immune function tests, were relatively normal. The same study also covered a second subject who had received a dosage equivalent to 2,900 times the normal level, who apparently suffered no notable negative effects other than chloracne. These patients were provided with olestra to accelerate dioxin elimination.
- In 2004, a notable individual case of dioxin poisoning, Ukranian politician Viktor Yushchenko was exposed to the second-largest measured dose of dioxins, according to the reports of the physicians responsible for diagnosing him. This is the first known case of a single high dose of TCDD dioxin poisoning, and was diagnosed only after a toxicologist recognised the symptoms of chloracne while viewing television news coverage of his condition.
- In the early 2000s, residents of the city of New Plymouth, New Zealand, report many illnesses of people living around and working at the Dow Chemical plant. This plant ceased production of 2,4,5-T in 1987.
- 1,995 people are suing DuPont, claiming dioxin emissions from its plant in DeLisle, Mississippi caused their cancers, illnesses or loved one's death. In August 2005, Glenn Strong, an oyster fisherman with the rare blood cancer multiple myeloma, was awarded $14 million from DuPont. In another case, parents claim dioxin from pollution caused the death of their 8 year old daughter. DuPont's DeLisle plant is one of three titanium dioxide facilities (including Edge Moor, DE and New Johnsonville, TN) that are the largest producers of dioxin in the country, according to the US EPA's Toxic Release Inventory.
Endosulfan is a neurotoxic organochlorine insecticide of the cyclodiene family of pesticides. It is highly toxic and an endocrine disruptor, and it is banned in several countries including Germany, Norway, and the Philippines. It is still used extensively in many countries including th US and India. Endosulfan is one of the more toxic pesticides on the market today, responsible for many fatal pesticide poisoning incidents around the world. Endosulfan is also a xenoestrogen, and it can act as an endocrine disruptor, causing reproductive and developmental damage in both animals and humans. Whether endosulfan can cause cancer is debated.
Endosulfan is acutely neurotoxic to both insects and mammals, including humans. The US EPA classifies it as Category I: "Highly Acutely Toxic" based on a LD50 value of 30 mg/kg for female rate, while the World Health Organisation classifies it as Class II "moderately Hazardous" based on a rat of LD50 of 80 mg/kg. It is a GABA-gated chloride channel antagonist and a Ca2+, Mg2+ ATpase inhibitor. Both of these enzymes are involved in the transfer of nerve impulses. Symptoms of acute poisoning include hyperactivity, tremors, convulsions, lack of coordination, staggering, difficulty breathing, nausea/vomiting, diarrhea, and in severe cases, unconsciousness. Doses as low as 35mg/kg have been documented to cause death in humans and many cases of sub-lethal poisoning have resulted in permanent brain damage. Farm workers with chronic endosulfan exposure are at risk of rashed and skin irritation.
EPA's acute reference dose for dietary exposure to endosulfan is 0.015mg/kg for adults and 0.0015 mg/kg for children. For chronic dietray exposure, the EPA reference doses are 0.006 mg/kg-day and 0.0006 mg/kg-day for adults and children, respectively. Endosulfan is listed as a known endocrine disruptor based on numerous in vitro studies documenting its estrogenic activity and animal studies demonstrating its reproductive and developmental toxicity especially among males.
Several studies have documented that endosulfan can also effect human development. Researchers studying children from a village in northern Kerala, India have linked endosulfan exposure to delays in sexual maturity among boys. The village is situated in a valley with a large cashew plantation in the hills above. Endosulfan - and only endosufan - had been applied aerially to the plantation for more than 20 years, and contaminated the village environment. The researchers compared the villagers to a control group of boys from a demographically similar village that lacked a history of endosulfan pollution. Relative to the control group, the exposed boys had high levels of endosulfan in their bodies, lower levels of testesterone, and delays in reaching sexual maturity. Birth defects of the male reproductive system including cryptorchidism were also more prevalent in the study group. The researchers concluded, "our study results suggest that endosulfan exposure in male children may delay sexual maturity and interfere with sex hormone synthesis." Increased incidences of cryptorchidism have been observed in other studies of endosulfan-exposed populations.
Endosulfan is not listed as known, probable, or possible carcinogen by the USEPA, IARC, or other agencies. There are no epidemiological studies linking exposure to endosulfan specifically to cancer in humans, but in vitro assays have shown that endosulfan can promote proliferation of human breast cancer cells. Evidence of carcinogenicity in animals is mixed.
According to the USEPA, endosulfan breaks down in to endosulfan sulfate and endosulfan diol, both of which have "structures similar to the parent compoud and are also of toxicological concern...The estimated half-lives for the combined toxic residues (endosulfan plus endosulfan sulfate) range from roughly 9 months to 6 years." The EPA concluded that, "based on environmental fate laboratory studies, terrestrial field dissipation studies, available models, monitoring studies, and published literature, it can be concluded that endosulfan is a very persistent chemical which may stay in the environment for lengthy periods of time, particularly in acid media." The EPA also concluded "endosulfan has relatively high potential to bioaccumulate in fish." The EPA recommends not more than 74ppb (part per billion) in lakes, streams, or rivers, and not more than 0.1 - 2ppm (parts per million) on surfaces of agricultural products.
The acute oral LD50 for rats is 70 mg (in aqueous suspension)/kg, 110mg (in oil)/kg and for dogs is 77 mg/kg. The acute subcutaneous LD50 for rabbits is 359mg (in oil)/kg, for male rats is >4000 and for female rats is 500 mg/kg. The inhalation LC50 (4 hour) for male rats is 0.0345 and for female rats is 0.0126 mg/L. The NOEL for rats (2 years) is 155 mg/kg diet and for dogs (1 year) is 10 mg/kg diet. The acute oral LD50 for mallard ducks is 205-245 and for ring-necked pheasants is 620-1000 mg/kg. Endosufan is highly toxic to fish and other aquatic life, the LC50 for golden orfe is 0.002mg/L water, daphnia (48 hour) is 75-750 ug/L and the EC50 (72 hours) for green algae is 0.56 mg/L. The Codex JMPR (1998) ADI is 0.006mg/kg body weight.
Lead is a chemical element in the periodic table that has the symbol Pb (Latin:plumbum) and atomic number 82. A soft, heavy, toxic and malleable metal, lead is bluish white when freshly cut but tarnishes to dull grey when exposed to air. Lead is used in building construction, lead-acid batteries, bullets and shot, and is part of solder, pewter, and fusible alloys. Lead has the highest atomic number of all stable elements and like mercury, another heavy metal, lead is a potent neurotoxin which accumulates in soft tisues and bone over time.
Lead is a poisonous metal that can damage nervous connections (especially in young children) and cause blood and brain disorders. Long term exposure to lead or its salts (especially soluble salts or the strong oxidant Pbo2 can cause nephropathy, and colic-like abdominal pains. The historical use of lead acetate (also known as sugar of lead) by the Roman Empire as a sweetener for wine is considered by some to be the cause of the dementia which affected many of the Roman Emperors. At one point in time, some lead compounds, because of their sweetness, were used by candy makers. Although this has been banned in industrialised nations, there was a 2004 scandal involving lead-laced Mexican candy being eaten by children in California. The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to schizophrenia). Lead-white paint has been withdrawn from sale in industrialised countries.
Lead salts used in pottery glazes have on occasion caused poisoning, when acid drinks, such as fruit juices, have leached lead ions out of the glaze. It has been suggested that what was known as "Devon colic" arose from the use of lead-lined presses to extract apple juice in the manufacture of cider. Lead is considered to be particularly harmful for women's ability to reproduce. For that reason many universities do not hand out lead-containing sampls to women for instructional laboratory analyses. Lead as a soil contaminant is a widespread issue, since lead may enter soil through (leaded) gasoline leaks from underground storage tanks or through a wastestream of lead paint or lead grindings from certain industrial operations.
Lead inhibits a-aminolevulinate (ALA) dehydratase and ferrochelatase, preventing both porphobilinogen formation and the incorporation of iron into protoporphyrin IX, the final step in heme synthesis. Inhibition of both of these steps results in ineffective heme synthesis and subsequent microcytic (hemoglobin-poor) anemia. The symptoms of chronic lead poisoning include neurological problems, such as reduced IQ, or nausea, abdominal pain, irritability, insomnia, metal taste in oral cavity, excess lethargy or hyperactivity, headache and, in extreme cases, seizure and coma. There are also associated gastrointestinal problems, such as constipation, diarrhea, vomiting, poor appetite, weight loss, which are common in acute poisoning. Other associated effects are anemia, kidney problems, and reproductive problems.
In humans, lead toxicity sometimes causes the formation of a bluish line along the gums, which is known as "Burton's line", although this is very uncommon in young children. Blood film examination may reveal "basophilic stippling" of red blood cells, as well as the changes normally associated with iron deficiency anemia (microcytosis and hypochromia). A direct link between early lead exposure and extreme learning disability has been confirmed by multiple researchers and child advocacy groups.
Lead has no known biological role in the body. The toxicity comes from its ability to mimic other biologically important metals, the most notable of which are calcium, iron and zinc. Lead is able to bind to and interact with the same proteins and molecules as these metals, but after displacement, those molecules function differently and fail to carry out the same reactions, such as in producing enzymes necessary for certain biological processes.
Most lead poisoning symptoms are thought to occur by interfering with an essential enzyme Delta-aminolevulinic acid dehydratase, or ALAD. ALAD is a zinc-binding protein which is important in the biosynthesis of heme, the cofactor found in hemoglobin. Lead poisoning also inhibits the enzyme ferrochelatase which catalyses the joining of protoporphyrin IX and Fe2+ to form a Heme. Lead also interferes with excitatory neurotransmission by glutamate, which is the transmitter at more than half the synapses in the brain and is critical for learning, The glutamate receptor thought to be associated with neuronal development and plasticity is the N-methyl-D-aspartate (NMDA) receptor, which is blocked selectively by lead. This disrupts long-term potentiation, which compromises the permanent retention of newly learned information.
It has been known for some time that lead is a patent inhibitor of the NMDA recpetor, a protein known to play an important role in brain development and cognition. Ezra Susser and his collegues at Columbia University in New York followed 12,000 children born in Oakland, California, between 1959 and 1966, whose mothers had given samples of blood serum while they were pregnant, which were frozen and stored for later analysis. They found that children who had been exposed to high levels of lead in the womb were more than twice as likely to go on to develop schizophrenia. Their research was presented at the 2004 annual meeting of the American Association for the Advancement of Science in Seattle, Washington.
Outside of occupational hazards, the majority of lead poisoning occurs in children under age twelve. The main sources of poisoning are from ingestion of lead contaminated soil (this is less of a problem in countries that no longer have leaded gasoline) and from ingestion of lead dust or chips from deteriorating lead-based paints. This is particularly a problem in older houses where the sweet-tasting lead paint is likely to chip, but deteriortaing lead-based paint can also powder and be inhaled. Small children also tend to teeth and suck on painted windowsills as they look outside. Lead has also been found in drinking water. It can come from plumbing and fixtures that are either made of lead or have trace amounts of lead in them.
One measure of lead in the body is the blood level (BLL), measured in micrograms of lead per deciliter of blood (ug/dL). Nearly everyone has a measurable BLL. The Centres for Disease Control and Prevention (CDC) states that a BLL of 10ug/dL or above is a cause for concern. However, lead can impair development even at BLLs below 10ug/dL. However, BLL measures current exposure to lead, but lead may also be incorporated into bone from prior exposures that will not show in BLLs until this bone-lead becomes "mobilised" through pregnancy or fracture healing. A fetus can be poisoned in utero if its mother had high bone-lead from either childhood exposure or a later occupational or other exposure that is subsequently mobilised by the fetal need for calcium. K-fluorscent X-ray metering can measure bone-lead.
The average person has less than 10 micrograms per decilitre, or 100 parts per billion, ppb, of lead in their blood. People who have been eposed to an unusual amount of lead will have blood lead levels higher than 200 ppb-most clinical symptoms of lead poisoning begin at around 100ppb. The effect on children's IQ has been noted at very low levels.
Mercury, also called quick silver, is a chemical element in the periodic table that has the symbol Hg (Latinised Greek: hydrargyrum, meaning watery or liquid silver) and atomic number 80. A heavy, silvery transition metal, mercury is one of five elements that are liquid at or near room temperature and pressure (the others are the metals caesium, francium, and gallium, and the non-metal bromine). Mercury is used in thermometres, barometres and other scientific apparartus, though concerns about the element's toxicity have led to mercury thermometres being largely phased out in clinical environments in favour if alcohol-filled, digital or thermistor-based instruments. It remains in use in a number of other ways in scientific and scientific research applications, and in dental amalgam. Mercury is mostly obtained by reduction from the mineral cinnabar.
From the mid-18th to the mid-19th centuries, a process called "carroting" was used in the making of felt hats. Animal skins were rinsed in an orange solution of the mercury compound mercuric nitrate Hg(NO3)2.2H2O. This process seperated the fur from the pelt and matted it together. This solution and the vapours it produced were highly toxic. Its use resulted in widespread cases of mercury poisoning among hatters. Symptoms included tremors, emotional lability, insomnia, dementia and hallucinations. The United States Public Health Service banned the use of mercury in the felt industry in December 1941. The psychological symptoms associated with mercury poisoning may have inspired the phrase "mad as a hatter".
Compounds of mercury tend to be much more toxic than the element itself, and organic compounds of mercury are often extremely toxic. Dimethylmercury, for example, is a potent neurotoxin that is lethal in amounts of a fraction of millilitre. Mercury damages the central nervous system, endocrine system, kidneys, and other organs, and adversely affects the mouth, gums and teeth. Exposure over long periods of time or heavy exposure to mercury vapour can result in brain damage and ultimately death. Mercury and its compounds are particularly toxic to foetuses and infants. Women who have been exposed to mercury in pregnancy have sometimes given birth to children with serious birth defects.
Some of the toxic effects of mercury are reversible, either through specific therapy or through natural elimination of the metal after exposure has been discontinued, However, heavy or prolonged exposure can do irreversible damage, particularly in fetuses, infants, and young children. Exposure to certain highly toxic compounds of mercury such as dimethylmercury can be fatal within hours or less. Mercury exposure in very young children can have severe neurological consequences, preventing nerve sheaths from forming properly. Research has been done that demonstrates the inhibitory effect that mercury has on myelin, the building block protein that forms these sheaths.
Mercury poisoning in the young is suspected as a possible cause of autistic behaviours, however there is a lack of quality peer-reviewed work on this matter and the claim of autism as mercury poisoning is considered suspect by mainstream medicine. Furthermore, the autistic community considers the theory offensive, as there is much evidence to suggest that autism is present from birth.
The mercury that is released in the environment ends up in surface water or soils eventually. When the pH values in acidic surface waters are between five and seven, the mercury concentrations in the water will increase. This is due to the mobilisation of mercury in the ground near a water source. Microorganisms are able to convert the mercury that reaches the surface water to methyl mercury and most organisms absorb this substance quickly. Methyl mercury is also known to cause nerve damage. Fish are among the organisms that absord methyl mercury in great amounts from water. As a consequence, methyl mercury accumulates in fish and passes into the food chain. The deleterious effects of mercury consumed by animals that eat fish include reproductive failure, damage to intestines, stomach disruption, DNA alteration, and kidney damage.
Cresols are organic compunds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compunds which are categorised as phenols (sometimes called phenolics). In its chemical structure, a cresol molecule has a methyl group substituted onto the benzene ring of a phenol molecule. There are three forms of cresols that are only slightly different in their chemical structure: ortho-cresol (o-cresol), meta-cresol (m-cresol), and para-cresol (p-cresol). Short-term and long term studies with animals have shown similar effects from exposure to cresols. No human or animal studies have shown harmful effects from cresols on the ability to have children. It is not known what the effects are from long-term ingestion or skin contact with low levels of cresols.
Polychlorinated biphenyls (PCBs) are a class of organic compound with 1 to 10 chlorine atoms attached to biphenyl and a general chemical formula of C12H10-xClx. Most PCBs were manufactured as cooling and insulating fluids for industrial transformers and capacitors. PCB production was banned in the 1970s due to the high toxicity of most PCB congeners and mixtures. PCB's are classified as persistent organic pollutants. Most of the 209 congeners of PCB are colourless, odourless crystals. The commercial mixtures are clear viscous liquids (the more highly chlorinated mixtures are more viscous, for example Aroclor 1260 is a "sticky resin"). Although the physical and chemical properties vary widely across the class, PCBs have low water solubilities and low vapour pressures. They are soluble in most organic solvents, oils and fats. PCBs are very stable compounds and do not degrade readily.
PCBs may be destroyed by chemical, thermal, and biochemical processes, though it is extremely difficult to achieve full destruction, and there is the risk of creating extremely toxic dibenzodioxins and dibenzofurans through partial oxidation. Because of the high thermodynamic stabilty of PCBs, all degradation mechanisms are difficult to sustain. Intentional degradation as a treatment of unwanted PCBs generally requires high heat or catalysis. Environmental and metabolic degradation generally proceeds quite slowly relative to most other compunds.
PCBs are persistent organic pollutants and have entered the environment through both use and disposal. The environmental transport of PCBs is complex and nearly global in scale. The public, legal, and scientific concerns about PCBs arose from research indicating they were likely carcinogens having the potential to adversely impact the environment and therefore undesirable as commercial products. Despite active research spanning five decades, extensive regulatory actions, and an effective ban on their production since the 1970s, PCBs still persist in the environment and remain a focus of attention.
The only North American producers, Monsanto, marketed PCBs under the trade name Aroclor from 1930 to 1977. These were sold under trade names followed by a 4 digit number. The first two digits generally refer to the number of carbon atoms in the biphenyl skeleton (for PCBs this is 12), the second two numbers indicate the percentage of chlorine by mass in the mixture. Thus Aroclor 1260 has 12 carbon atoms and contains 60% chlorine by mass. An exception is Arochlor 1016, which also has 12 carbon atoms, but has 42% chlorine by mass. PCB mixtures have been used for a variety of applications, including dielectric fluids for capacitors and transformers, heat transfer fluids, hydraulic fluids, lubricating and cutting oils, and as additives in pesticides, paints, carbonless copy ("NCR") paper, adhesives, sealants, plastics, reactive flame retardants, and as a fixative for microscopy. There were also used in surgical implants.
Manufacture peaked in the 1960s, by which time the electrical industry had lobbied congress to make them mandatory safety equipment, knowing all the while that they were extremely toxic. In 1966, they were discovered by Swedish chemist Dr. Soren Jensen as an environmental contaminant, and according to a 1994 article in Sierra, it was Dr. Jensen who named them. Previously, they had been called "phenols" or referred to by various trade names, such as Aroclor, Kennechlor, Pyrenol and others. Their commercial utility was based largely on their chemical stability, including low flammability, and desirable physical properties, including electrical insulating properties. Their chemical and physical stability has also been responsible for their continuing persistence in the environment, and the lingering interest decades after regulations were imposed to control environmental contamination.
The General Electric Co. discharged between 209,000 and 1.3 million pounds (94,800 and 590,000 kg) of PCBs into the Hudson River from two capacitor manufacturing plants located in Hudson Falls, New York and Fort Edward, New York. Since that time, the spread of PCBs throughout the river and its food chain has created an extensive toxic waste problem. About 200 miles of the river is designated as a Superfund site. In 1976, because of the concern over the bioaccumulation of PCBs in fish and other aquatic organisms and their subsequent consumption by people, the State of New York banned fishing in the Upper Hudson River and commerfcial fishing of striped bass, and several other species, in the Lower Hudson. In August 1995, the Upper Hudson was re-opened to fishing, but only on a catch and release basis.
From the late 1950s through 1977, Westinghouse Electric used PCBs in the manufacture of capacitors in its Bloomington, Indiana plant. Reject capacitors were hauled and dumped in area salvage yards and landfills. Workers also dumped PCB oil down factory drains that contaminated the city sewage treatment plant. The City of Bloomington gave away the sludge to area farmers and gardeners, creating anywhere from 200 to 2000 sites which remain unaddressed. Over 2,000,000 pounds of PCBs were estimated to have been dumped in Monroe and Owen Counties, making it the biggest concentration of PCBs in the world. Although federal and state authorities have been working on the site remediation, many areas remain contaminated. Concerns have been raised regarding the removal of PCBs from the karst limestone topography, and regarding the possible disposal options. To date, the Westinghouse Bloomington PCB Superfund site case does not have a RI/FS (Remedial Investigation/Feasibility Study) and ROD (Record of Decision), although Westinghouse signed a US Department of Justice Consent Decree in 1985.
PCBs have been detected globally, from the most urbanised areas that are the centres of PCB pollution, to regions north of the arctic circle. Typical urban atmospheric concentrations are in the picogram per cubic metre range. The atmosphere serves as the primary route for global transport of PCBs, particularly for those congeners with 1 to 4 chlorine atoms. The toxicity of PCBs to animals was first noticed in the 1970s when emaciated seabird corpses with very high PCB body burdens were washed up on beaches. The source(s) of the PCBs was (were) unknown though, because seabirds may die at sea and be washed ashore from a very wide area. Where thet were found was no reliable indicator of where thet had died.
The toxicity of PCBs varies considerably among congeners. The coplanar PCBs, known as non-ortho PCBs because they are not substituted at the ring positions ortho to (next to) the other rings, (ie PCBs 77, 126, 169, etc), tend to have dioxin-like properties, and generally are among the most toxic congeners. Because PCBs are almost invariably found in complex mixtures, the concept of toxic equivalency factors (TEFs) has been developed to facilitate risk assessment and regulatory control, where more toxic PCB congeners are assigned higher TEF values. One of the most toxic compounds known, 2,3,7,8-tetrachlorodibenzo[p]dioxin, is assigned a TEF of 1.
The most commonly observed health effects in people exposed to large amounts of PCBs are skin conditions such as chloracne and rashes, but these awere known to be symptoms of systemic poisoning dating back to the 1920s. Studies in exposed workers have shown changes in blood and urine that may indicate liver damage. PCB exposures in the general population are not likely to result in skin and liver effects. Most of the studies of health effects of PCBs in the general population examined children of mothers who were exposed to PCBs.
Animals that ate food containing large amounts of PCBs for short periods of time had mild liver damage and some died. Animals that ate smaller amounts of PCBs in food over several weeks or months developed various kinds of health effects, including anaemia; acne-like skin conditions (chloracne); and liver, stomach, and thyroid gland injuries (including hepatocarcinoma). Other effects of PCBs in animals include changes in the immune system, behavioural alterations, and impaired reproduction. PCBs are not known to cause birth defects in humans, although those that have dioxin-like activity are known to cause a variety of teratogenic effects in animals.
Women who were exposed to relatively high levels of PCBs in the workplace or ate large amounts of fish contaminated with PCBs had babies that weighed slightly less than babies from women who did not have these exposures. Babies born to women who ate PCB-contaminated fish also showed abnormal responses in tests of infant behaviour. Some of these behaviours, such as problems with motor skills and a decrease in short-term memory, lasted for several years. Other studies suggest that the immune system was affected in children born to and nursed by mothers exposed to increased levels of PCBs. The most likely way infants will be exposed to PCBs is from breast milk. Transplacental transfers of PCBs were also reported. Because an infant will receive more than ten times the amount of PCBs from breast milk than it will for the rest of its life, it is being debated whether the benefits of breast-feeding are greater than the risks from exposure to PCBs.
Studies have shown that PCBs alter estrogen levels in the body and contribute to reproduction problems. In the womb, males can be feminised or the baby may be intersex, neither male nor a female. Also, both sets of reproductive organs may develop. More instances of this are being reported. Biological magnification of PCBs has also led to polar bears and whales that have both male and female sex organs and males than cannot reproduce. This effect is also known as endocrine disruption. Endocrine Disrupting Chemicals (EDC's) pose a serious threat to reproduction in top-level predators.
A few studies of workers indicate that PCBs were associated with specific kinds of cancer in humans, such as cancer of the liver and biliary tract. Rats that ate food containing high levels of PCBs for two years developed liver cancer. The USA Department of Health and Human Services (DHHS) has concluded that PCBs may reasonably be anticipated to be carcinogens. The US Environmental Protection Agency (USEPA) and the International Agency for Research on Cancer (IARC) have determined that PCBs are probably carcinogenic to humans. PCBs are also classified as probable human carcinogens by the National Cancer Institute, World Health Organisation and the Agency for Toxic Substances and Disease Registry. Recent research by the National Toxicology Program has confirmed that PCB126 (Technical Report 520) and a binary mixture of PCB126 and PCB 153 (Technical Report 531) are carcinogens.
As discussed, PCBs exhibit a wide range of toxic effects. These effects may vary depending on the specific PCB. Toxicity of coplanar PCBs (such as dioxin) and mono-ortho-PCBs may be primarily mediated via binding to aryl hydrocarbon receptor (AhR). Because AhR is a transcription factor abnormal activation may disrput cell function by latering the transcription of genes. The concept of toxic equivalency factors (TEF) is based on the ability of a PCB to activate AhR. However, not all effects may be mediated by the AhR receptor. For example, di-ortho-substituted non-coplanar PCBs interfere with intracellular signal transduction dependent on calcium; this may lead to neurotoxicity. Ortho-PCBs may disrupt thyroid hormone transprt by binding to transthyretin.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are chemical compounds that consist of fused aromatic rings that do not contain heteroatoms or carry substituents. These compounds can be point source pollutants (eg oil spill) or non-point source (eg atmospheric deposition) and are one of the most widespread organic pollutants. Some of them are known as suspected carcinogens, and are linked to other health problems. They are primarily formed by incomplete combustion of carbon-containing fuels such as wood, coal, diesel, fat or tobacco. Tar also contains PAHs. Since human civilisation relies so heavily on combustion, PAHs are inevitably linked to our energy production. In this sense, PAH can be thought of as marker molecules as their abundance can be directly proportional to combustions processes in the region and therefore directly related to air quality. Different types of combustion yield different distributions of PAHs. Thus, those produced from coal burning are different than those produced by motor-fuel combustion, which differ from those produced by forest fires. Some PAHs occur within crude oil, arising from chemical conversion of natural product molecules, such as steroids, to aromatic hydrocarbons.
PAHs of three rings or more have low solubilities in water and a low vapour pressure. As molecular weight increases, aqueous solubility and vapour pressure decrease. The aqueous solubility decreased approximately one order of magnitude for each additional rings. PAHs with two rings are more soluble in water and more volatile. Because of these properties, PAHs in the environment are found primarily in soil and sediment, as opposed to in water or air. PAHs, however, are also often found in particles suspended in water and air. Natural crude oil and coal deposits contain significant amounts of PAHs, as do combustion products and smoke from naturally occurring forest fires.
PAHs toxicity is very structurally dependent, with isomers (PAHs with the same formula and number of rings) varying from being nontoxic to being extremely toxic. Thus, highly carcinogenic PAHs may be small or large. One PAH compound, benzo[a]pyrene, is notable for being the first chemical carcinogen to be discovered (and is one of many carcinogens found in cigarette smoke). The EPA has classified seven PAH compunds as probably human carcinogens: benzo[a]anthrocene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthese, chyrsene, dibenz[a,h]anthracene and indeno[1,2,3-cd]pyrene.
Worksafe Australia has regulated for naphthalene that the eight hour time weighted average (TWA) exposure limit is 10 parts per million, with the short term exposure limit (STEL) concentration not to exceed 15 parts per million. Australian Drinking Water Quality Guidelines (NHMRC and ARMCANZ, 1996): Benzo-(a)-pyrene: Maximum of 0.00001mg/L (i.e. 0.00000001g/L).
Polycyclic aromatic hydrocarbons have moderate to high acute (short-term) toxicity to aquatic life and birds. Some cause damage and death to agricultural and ornamental crops. They have moderate to high chronic (long-term) toxicity to aquatic life. Insufficient data are available on the acute or chronic toxicity to land animals. Polycyclic aromatic hydrocarbons are moderately persistent in the environment, and can bioaccumulate. The concentrations of polycyclic aromatic hydrocarbons found in fish and shellfish is expected to be much higher than the environment from which it is taken.
Selenium is a chemical element with atomic number 34, with the chemical symbol Se. Selenium occurs only rarely in the free state of nature. It is a nonmetal that is chemically related to sulfur and tellurium. It is toxic in large amounts, but trace amounts of it, forming the active center of certain enzymes, are necessary for the function of all cells in (probably) all animals. Selenium requirements in plants differ by species, with some plants apparently requiring none. Although selenium is an essential trace element it is toxic if taken in excess. Exceeding the Tolerable Upper Intake Level of 400 micrograms per day can lead to selenosis. Symptoms of selenosis include a garlic odour on the breath, gastrointestinal disorders, hair loss, sloughing of nails, fatigue, irritability and neurological damage. Extreme cases of selenosis can result in cirrhosis of the liver, pulmonary edema and death.
Elemental selenium and most metallic selenides have relatively low toxicities because of their low bioavailability. By contrast, selenate and selenite are very toxic, and have modes of action similar to that of arsenic. Hydrogen selenide is an extremely toxic, corrosive gas. Selenium also occurs in organic compunds such as dimethyl selenide, selenomethionine and selenocysteine, all of which have high bioavailability and are toxic in large doses. Selenium poisoning of water systems may result whenever new agricultural runoff courses through normally-dry undeveloped lands. This process leaches natural soluble selenium compounds (such as selenates) into the water, which may then be concentrated in new "wetlands" as it evaporates. High selenium levels produced in this fashion have been found to have caused certain congenital disorders in wetland birds.
Tributyltin (TBT, Bu3SnH) is a trialkyl organotin compound. Tributyltin compounds are a subgroup of the trialkyl organotin family of compounds. They are the main active ingredients in biocides used to control a broad spectrum of organisms. Uses include wood preservation and preservation, antifouling of boats (in marine paints), antifungal action in textiles and industrial water systems, such as cooling tower and refrigeration water systems, wood pulp and paper mill systems, and breweries. It is also used for control of schistosomiassis in various parts of the world. Tributyltin oxide is the widely used compound in TBT containing commercial products. TBT compounds are considered as toxic chemicals which have negative effects on humans and the environment. Tributyltin compounds are moderately to highly persistent organic pollutants. One common example is leeching of TBT from marine paints into the aquatic environment, causing irreversible damage to the aquatic life.
TBT is harmful to some marine organisms, including the dog-whelk. TBT causes dog whelks to suffer from imposex: females develop male sexual characteristics such as a penis. This causes them to become infertile or even die. In severe cases males can develop egg sacs. Studies have shown that wild, dead, sea otters (Enhydra lutra) and stranded bottlenose dolphins can have extremely high levels of tributyltin in their livers. Both tributyltin and dibutyltin, a metabolite byproduct, cause immunosuppression, leading to secondary infections. This was supported by the finding that otters dying of infectious causes tend to have higher levels of tissue butyltins than those dying of trauma of other causes. Butyltin residues in Southern sea otters (Enhydra lutris nereis) were found dead along California coastal waters. Environ. Sci. Technol, 32:1169-1175).
TBT is extremely toxic to molluscs. In the case of bivalve larvae, the effective concentrations (EC50) of TBT is 1000 times lower than that of any other toxic compound introduced into the marine environment. (see Fig.15 on p 134 in: His et al. 1999. The assessment of marine pollution - bioassays with marine embryos and larvae. Adv Mar Biol 37:3-178). TBT also causes imposex in marine gastropods and is probably responsible for reductions in their populations in zones with important ship traffic. It is reported to be an endocrine and immune system toxicant and ranked as 'most hazardous (worst 10%) to ecosystems by the USEPA.
USEPA Aquatic Life Criteria for Tributyltin (TBT)
Except possibly where a locally important species is very sensitive, saltwater aquatic life and their uses should not be affected unacceptably if the one-hour average concentration of TBT does not exceed 0.42ug/L more than once every three years on the average (acute criterion) and if the four-day average concentration of TBT does not exceed 0.0074 ug/L more than once every three years on the average (chronic criterion).
Lindsay Swinden PhD